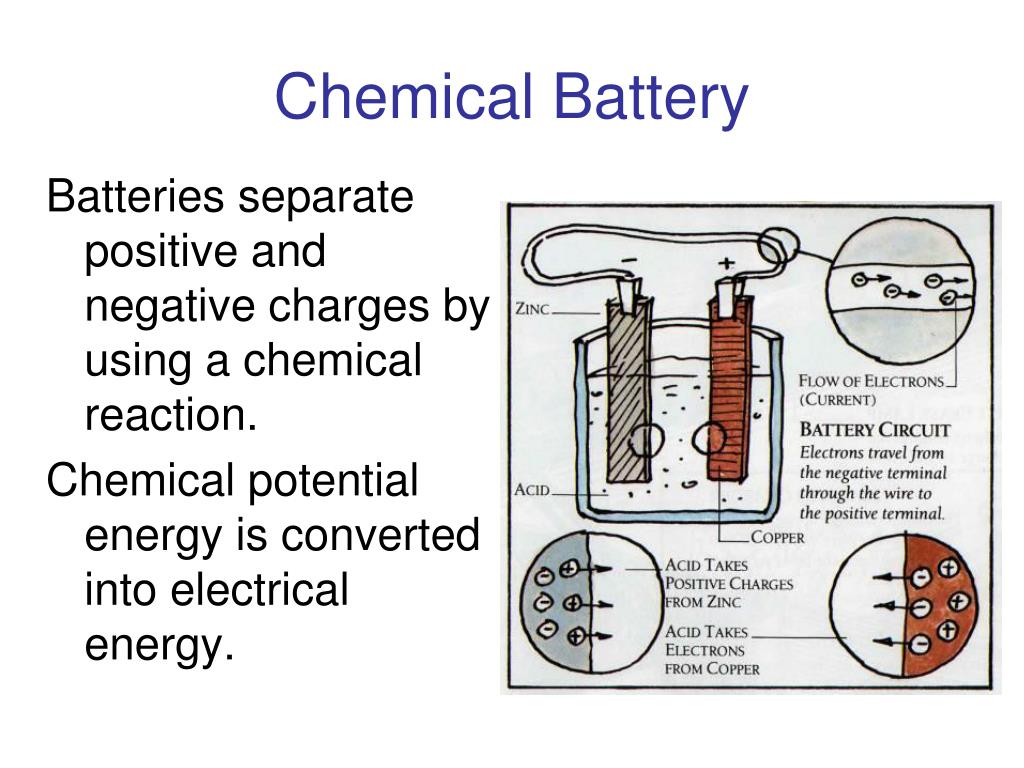
Battery Charging Chemical Equation . The method of regenerating active material is called. The analysis provides an explanation of basic electrochemistry that will help students better understand. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. Oxidation happens at the anode, where the material loses electrons. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. The overall chemical equation for this type of battery is as follows: There are huge chemical process is involved in lead acid battery’s charging and discharging condition. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The chemical process of extracting current from a secondary battery (forward reaction) is called discharging.
from www.slideserve.com
There are huge chemical process is involved in lead acid battery’s charging and discharging condition. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The analysis provides an explanation of basic electrochemistry that will help students better understand. The overall chemical equation for this type of battery is as follows: Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. Oxidation happens at the anode, where the material loses electrons. The method of regenerating active material is called.
PPT Chapter 23 Electric Current PowerPoint Presentation ID163039
Battery Charging Chemical Equation The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. Oxidation happens at the anode, where the material loses electrons. There are huge chemical process is involved in lead acid battery’s charging and discharging condition. The overall chemical equation for this type of battery is as follows: The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. The method of regenerating active material is called. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The analysis provides an explanation of basic electrochemistry that will help students better understand.
From orvelleblog.blogspot.com
Spice of Lyfe Lead Acid Battery Chemical Reaction Equation Battery Charging Chemical Equation \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. The analysis provides an explanation of basic electrochemistry that will help students better understand. The chemical process of extracting current from a secondary battery (forward reaction) is. Battery Charging Chemical Equation.
From www.slideserve.com
PPT Chapter 23 Electric Current PowerPoint Presentation ID163039 Battery Charging Chemical Equation There are huge chemical process is involved in lead acid battery’s charging and discharging condition. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. This is represented by the equation \[t =. Battery Charging Chemical Equation.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID321506 Battery Charging Chemical Equation This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. The method of regenerating active material is called. The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. Charging is the process of restoring a battery’s energy by reversing. Battery Charging Chemical Equation.
From skill-lync.com
Week 1 Understanding Different Battery Chemistry SkillLync Battery Charging Chemical Equation This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. The analysis provides an explanation of basic electrochemistry that. Battery Charging Chemical Equation.
From instrumentationtools.com
Discharge and Charging of LeadAcid Battery Inst Tools Battery Charging Chemical Equation The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. There are huge chemical process is involved in lead acid battery’s charging and discharging condition. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The analysis provides an explanation of basic electrochemistry that will help students better understand. This is represented by the equation \[t = \dfrac{q_p}{k^k}. Battery Charging Chemical Equation.
From www.researchgate.net
Schematic diagram of the Vanadium Redox flow battery and the reaction Battery Charging Chemical Equation The method of regenerating active material is called. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. The chemical process of. Battery Charging Chemical Equation.
From nanohub.org
Courses nanoHUBU Introduction to the Materials Science Battery Charging Chemical Equation The analysis provides an explanation of basic electrochemistry that will help students better understand. There are huge chemical process is involved in lead acid battery’s charging and discharging condition. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. Charging is the process of restoring a battery’s energy. Battery Charging Chemical Equation.
From www.animalia-life.club
Using A Chemical Battery Chemical Change Battery Charging Chemical Equation \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The method of regenerating active material is called. There are huge chemical process is involved in lead acid battery’s charging and discharging condition. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. The overall chemical equation. Battery Charging Chemical Equation.
From www.nvcuk.com
Introduction to Lithium Batteries Battery Charging Chemical Equation The analysis provides an explanation of basic electrochemistry that will help students better understand. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. There are huge chemical process is involved in lead acid battery’s charging and discharging condition. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. This is represented by the equation \[t = \dfrac{q_p}{k^k}. Battery Charging Chemical Equation.
From www.slideshare.net
Alkaline Battery Battery Charging Chemical Equation \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The overall chemical equation for this type of battery is as follows: The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. Oxidation happens at the anode, where the material loses electrons. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. Charging. Battery Charging Chemical Equation.
From www.toppr.com
23. A battery is constructed of Cr and Na, Cr,0,. The unbalanced Battery Charging Chemical Equation The overall chemical equation for this type of battery is as follows: Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. The method of regenerating active material is called. The analysis provides an explanation of basic electrochemistry that will help students better understand. This. Battery Charging Chemical Equation.
From simanaitissays.com
LITHIUMAIR—THE BEST OF BATTERIES, THEORETICALLY Simanaitis Says Battery Charging Chemical Equation \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. Oxidation happens at the anode, where the material loses electrons. The overall chemical. Battery Charging Chemical Equation.
From www.animalia-life.club
Using A Chemical Battery Chemical Change Battery Charging Chemical Equation The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. The method of regenerating active material is called. The analysis provides an explanation of basic electrochemistry that will help students better understand. The overall chemical equation for this type of battery is as follows: \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The chemical process of. Battery Charging Chemical Equation.
From www.youtube.com
Electrochemical Reaction in Lithium Ion Battery YouTube Battery Charging Chemical Equation The method of regenerating active material is called. Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. Oxidation happens at the anode, where the material loses electrons. This is. Battery Charging Chemical Equation.
From skill-lync.com
Understanding Different Battery Chemistry SkillLync Battery Charging Chemical Equation The method of regenerating active material is called. Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. There are huge chemical process is involved in lead acid battery’s charging and discharging condition. The analysis provides an explanation of basic electrochemistry that will help students. Battery Charging Chemical Equation.
From batteryworld.varta-automotive.com
How does a car battery work and how is it constructed? Battery Charging Chemical Equation Oxidation happens at the anode, where the material loses electrons. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. The method of regenerating active material is called. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The overall chemical equation for this type of battery. Battery Charging Chemical Equation.
From panther.ph
Battery Charging 101 Understanding battery chemistry and the battery Battery Charging Chemical Equation The overall chemical equation for this type of battery is as follows: This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the.. Battery Charging Chemical Equation.
From www.electroniclinic.com
Lead acid battery, Construction and, Working, and Charging Battery Charging Chemical Equation Oxidation happens at the anode, where the material loses electrons. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber. Battery Charging Chemical Equation.
From www.researchgate.net
The principle of the lithiumion battery (LiB) showing the Battery Charging Chemical Equation This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. The overall chemical equation for this type of battery is as follows: Oxidation happens at the anode, where the. Battery Charging Chemical Equation.
From www.youtube.com
How Does a Battery Work? Alkaline Batteries AP Chemistry YouTube Battery Charging Chemical Equation The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. The analysis provides an explanation of basic electrochemistry that will help students better understand. The overall chemical equation for this type of battery is as follows: The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. Oxidation happens at the. Battery Charging Chemical Equation.
From www.google.co.ug
Patent EP1533856A1 Alphaleaddioxide coated electrode grid for lead Battery Charging Chemical Equation This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. Oxidation happens at the anode, where the material loses electrons. The overall chemical equation for this type of battery is as follows: The analysis provides an explanation of basic electrochemistry that will help students. Battery Charging Chemical Equation.
From www.youtube.com
Basic Chemistry and Lead Acid Batteries YouTube Battery Charging Chemical Equation The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. Oxidation happens at the anode, where the material loses electrons. There are huge chemical process is involved in lead. Battery Charging Chemical Equation.
From www.meritnation.com
Write chemical equation for discharging and recharging of lead storage Battery Charging Chemical Equation The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. The method of regenerating active material is called. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The analysis provides an. Battery Charging Chemical Equation.
From www.e-batterystore.com
The principle and thermodynamics of leadacid batteries e Battery Charging Chemical Equation There are huge chemical process is involved in lead acid battery’s charging and discharging condition. The analysis provides an explanation of basic electrochemistry that will help students better understand. The method of regenerating active material is called. The overall chemical equation for this type of battery is as follows: Oxidation happens at the anode, where the material loses electrons. The. Battery Charging Chemical Equation.
From www.youtube.com
Electrochemistry / Secondary Cells /Nickel Cadmium battery/Leadacid Battery Charging Chemical Equation There are huge chemical process is involved in lead acid battery’s charging and discharging condition. The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. The analysis provides an explanation of basic electrochemistry that will help students better understand. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. Oxidation. Battery Charging Chemical Equation.
From www.toppr.com
Identify the compound D. Chemistry Questions Battery Charging Chemical Equation The method of regenerating active material is called. There are huge chemical process is involved in lead acid battery’s charging and discharging condition. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about. Battery Charging Chemical Equation.
From nanohub.org
Courses nanoHUBU Introduction to the Materials Science Battery Charging Chemical Equation The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. The method of regenerating active material is called. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The analysis provides an explanation. Battery Charging Chemical Equation.
From www.electricity-magnetism.org
Battery Principle of operation Electricity Battery Charging Chemical Equation There are huge chemical process is involved in lead acid battery’s charging and discharging condition. The analysis provides an explanation of basic electrochemistry that will help students better understand. Oxidation happens at the anode, where the material loses electrons. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. The method of regenerating active material. Battery Charging Chemical Equation.
From www.animalia-life.club
Using A Chemical Battery Chemical Change Battery Charging Chemical Equation This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The analysis. Battery Charging Chemical Equation.
From www.askiitians.com
Write the chemistry of recharging the lead storage battery? askIITians Battery Charging Chemical Equation \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. The analysis provides an explanation of basic electrochemistry that will help students better understand. Oxidation happens at the anode, where the material loses electrons. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is. Battery Charging Chemical Equation.
From eesasha.com
Capacity of a Battery Charge vs Energy Stored eeSasha Electrical Battery Charging Chemical Equation The method of regenerating active material is called. The overall chemical equation for this type of battery is as follows: The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. The analysis provides an explanation of basic electrochemistry that will. Battery Charging Chemical Equation.
From theconversation.com
How do lithiumion batteries work? Battery Charging Chemical Equation Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. The method of regenerating active material is called. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. The. Battery Charging Chemical Equation.
From www.slideserve.com
PPT Batteries PowerPoint Presentation, free download ID1850273 Battery Charging Chemical Equation There are huge chemical process is involved in lead acid battery’s charging and discharging condition. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The method of regenerating active material is called. The diluted sulfuric acid h 2 so 4 molecules break into two parts when the. The analysis provides an explanation of basic electrochemistry that will help students better understand. The. Battery Charging Chemical Equation.
From www.altestore.com
deepcyclebatterieschargingdischargingchemistry altE DIY Solar Blog Battery Charging Chemical Equation \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The overall chemical equation for this type of battery is as follows: Oxidation happens at the anode, where the material loses electrons. The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current,. Battery Charging Chemical Equation.
From www.nagwa.com
Question Video Identifying the Overall Equation for the Reaction That Battery Charging Chemical Equation Charging is the process of restoring a battery’s energy by reversing the discharge reactions, while discharging is the release of stored energy through chemical reactions. This is represented by the equation \[t = \dfrac{q_p}{k^k} \nonumber \] where i is the current, k is a constant of about 1.3, t is the. \[nio(oh)_{(s)} + mh \rightarrow ni(oh)_{2(s)} + m_{(s)}. The diluted. Battery Charging Chemical Equation.