Ph Method Verification . This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. In the omcl context, pharmacopoeial methods and validated methods. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. Those requirements have implications on the calibration and verification standards used. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method.
from instrumentationtools.com
Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. Those requirements have implications on the calibration and verification standards used. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. In the omcl context, pharmacopoeial methods and validated methods. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method.
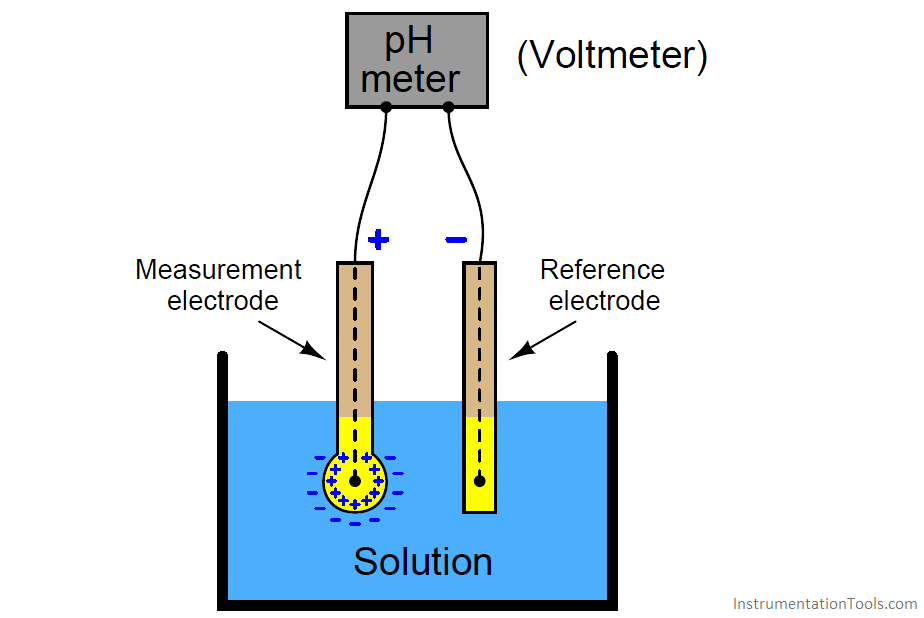
pH measurement Instrumentation Tools
Ph Method Verification In the omcl context, pharmacopoeial methods and validated methods. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. Those requirements have implications on the calibration and verification standards used. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. In the omcl context, pharmacopoeial methods and validated methods. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the.
From www.researchgate.net
Deming regression analysis for Thermo Fisher pH Chemical method vs. pH... Download Scientific Ph Method Verification Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. In the omcl context, pharmacopoeial methods and validated methods. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. When a method is verified, the laboratory is required to demonstrate. Ph Method Verification.
From www.dreamstime.com
Scale of Ph Value for Acid and Alkaline Solutions, Infographic Acidbase Balance. Scale for Ph Method Verification When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. Verification is measuring. Ph Method Verification.
From www.researchgate.net
Testing methods of the different pH dyed sample. Download Scientific Diagram Ph Method Verification With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. Those requirements have implications on the calibration and verification standards used. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. In the omcl context, pharmacopoeial methods and validated methods. Verification is measuring a standard that does not. Ph Method Verification.
From www.youtube.com
pH metric titration of Strong Acid and Strong Base Estimation of the strength of HCl by pH Ph Method Verification Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. In the omcl context, pharmacopoeial methods and validated methods. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. When a method is verified, the laboratory is required to demonstrate. Ph Method Verification.
From acidsandbasesrios.weebly.com
pH Acids and Bases Ph Method Verification With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Verification of a test method demonstrates that the laboratory. Ph Method Verification.
From www.gerhardt.de
Advantages of pH measurement with pH electrode compared to colorimetric detection C. Gerhardt Ph Method Verification In the omcl context, pharmacopoeial methods and validated methods. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Verification is measuring a standard that does not form part of the calibration set to ensure. Ph Method Verification.
From sciencenotes.org
How to Calculate pH Formula and Examples Ph Method Verification Those requirements have implications on the calibration and verification standards used. In the omcl context, pharmacopoeial methods and validated methods. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. Usp outlines. Ph Method Verification.
From www.researchgate.net
Illustration of the concept of the pH electrode method for measuring... Download Scientific Ph Method Verification Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. Those requirements have implications on the calibration and verification standards used. With the introduction of en. Ph Method Verification.
From smartgardenguide.com
What Is The Best pH For Hydroponics? Smart Garden Guide Ph Method Verification This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. Those requirements have implications on the calibration and verification standards used. When a method is verified, the laboratory is. Ph Method Verification.
From qcqa.industrialguide.co.in
SOP for Calibration of pH Meter Temperature Sensor Ph Method Verification Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. With the introduction of en iso/iec 17025,. Ph Method Verification.
From www.researchgate.net
Determination of pH pzc of Nb 2 O 5 catalyst by the pH drift method [28] Download Scientific Ph Method Verification Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. In the omcl context, pharmacopoeial methods and validated methods. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Those requirements have implications on the calibration and verification standards used. With the. Ph Method Verification.
From www.newagenutrients.com
pH Scale newagenutrients Ph Method Verification Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. In the omcl context, pharmacopoeial methods and. Ph Method Verification.
From okrainmygarden.com
4 Ways to Raise Soil pH (Make it More Alkaline) Okra In My Garden Ph Method Verification Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. Those requirements have implications on the calibration and verification standards used. In the omcl context, pharmacopoeial methods and validated methods. Usp outlines. Ph Method Verification.
From www.researchgate.net
Validation results of pH simulations with measurements values for an... Download Scientific Ph Method Verification In the omcl context, pharmacopoeial methods and validated methods. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. Verification. Ph Method Verification.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Ph Method Verification Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. Those requirements have implications on the calibration and verification standards used. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. With the. Ph Method Verification.
From blog.juicegrape.com
how to test for pH Archives Musto Wine Grape Company, LLC. BlogMusto Wine Grape Company, LLC Ph Method Verification With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. Those requirements have implications on the calibration and verification standards. Ph Method Verification.
From control.com
Colorimetric and Potentiometric pH Measurement Introduction to Continuous Analytical Ph Method Verification With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. In the omcl. Ph Method Verification.
From www.semanticscholar.org
Table 2 from pH Testing as the Primary Method for Nasogastric Tube Placement Verification Ph Method Verification Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. Verification of a test. Ph Method Verification.
From growfully.com
The Easiest and Most Reliable Way to Test Soil pH Growfully Ph Method Verification Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. Those requirements have implications on the calibration and verification standards used. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including. Ph Method Verification.
From www.researchgate.net
Verification of properties of pH nanoswitch. (a) Schematic diagram of... Download Scientific Ph Method Verification Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. In the omcl context, pharmacopoeial methods and validated methods. Those requirements have implications on the calibration and verification standards used.. Ph Method Verification.
From www.creeklinehouse.com
How to Test Soil pH Levels The Creek Line House Ph Method Verification Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. Those requirements have implications on the calibration and verification standards used. This sop is applicable for performing analytical method verification. Ph Method Verification.
From enggyd.blogspot.com
Engineers Guide pH Measuring Instruments, Working Principle, Theory and Determinating Methods Ph Method Verification Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. In the omcl context, pharmacopoeial methods and validated methods. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. Verification. Ph Method Verification.
From www.researchgate.net
Analytical validation parameters for CLO and PH by HPTLC method Download Table Ph Method Verification Those requirements have implications on the calibration and verification standards used. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. This sop is applicable for. Ph Method Verification.
From www.researchgate.net
Chromatographic Conditions for the Official Ph. Eur. Method Download Scientific Diagram Ph Method Verification Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. In the omcl context, pharmacopoeial methods and validated methods. Verification. Ph Method Verification.
From www.researchgate.net
(A) Schematic and photographs of the pHdriven process along with a... Download Scientific Diagram Ph Method Verification Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. Those requirements have implications on the calibration. Ph Method Verification.
From mmerevise.co.uk
pH Curves Questions and Revision MME Ph Method Verification This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Those requirements have implications on the calibration and verification standards used. In the omcl context, pharmacopoeial methods and validated methods. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. With the introduction of en. Ph Method Verification.
From www.youtube.com
How To Test Soil PH YouTube Ph Method Verification This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. In the omcl context, pharmacopoeial methods and validated methods. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. Those requirements have implications on the calibration and verification standards used. Verification is measuring a standard. Ph Method Verification.
From www.chegg.com
1 Data Activity 1 Data Table pH Determination Well A1 Ph Method Verification When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality.. Ph Method Verification.
From www.mvchamber.org
จำหน่าย pH Meter เครื่องวัดกรดด่างคุณภาพสูง ขายราคาถูก Ph Method Verification In the omcl context, pharmacopoeial methods and validated methods. Those requirements have implications on the calibration and verification standards used. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is accurate, and the standard reads. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode.. Ph Method Verification.
From instrumentationtools.com
pH measurement Instrumentation Tools Ph Method Verification Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. In the omcl context, pharmacopoeial methods and validated methods. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. Those requirements have implications on the calibration and verification standards used. This sop is. Ph Method Verification.
From forumautomation.com
Maintaining pH Meter Accuracy Importance and Methods of Verification Analytical Instruments Ph Method Verification With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. In the omcl context, pharmacopoeial methods and validated methods. Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. Verification is measuring a standard that does not form part of the calibration set to ensure that the calibration is. Ph Method Verification.
From control.com
Colorimetric and Potentiometric pH Measurement Introduction to Continuous Analytical Ph Method Verification With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Usp outlines the requirements of the ph measuring system,. Ph Method Verification.
From study.com
pH Determination Overview & Methods Lesson Ph Method Verification Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be completed before the. In the omcl context, pharmacopoeial methods and validated methods. Those requirements have implications on the calibration and verification standards used. This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. Usp outlines. Ph Method Verification.
From www.youtube.com
A Simple Guide to the pH Scale and Indicators YouTube Ph Method Verification Usp outlines the requirements of the ph measuring system, including the ph meter and electrode. When a method is verified, the laboratory is required to demonstrate that it can achieve certain specific performance characteristics/parameters. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. Verification is measuring a standard that does not form part. Ph Method Verification.
From www.processplus.co.uk
Instrument verification for pH & temperature instruments. Ph Method Verification This sop is applicable for performing analytical method verification of compendial procedure/validated analytical methods in quality. With the introduction of en iso/iec 17025, the requirements governing the documentation of methods, including method. Those requirements have implications on the calibration and verification standards used. In the omcl context, pharmacopoeial methods and validated methods. Usp outlines the requirements of the ph measuring. Ph Method Verification.