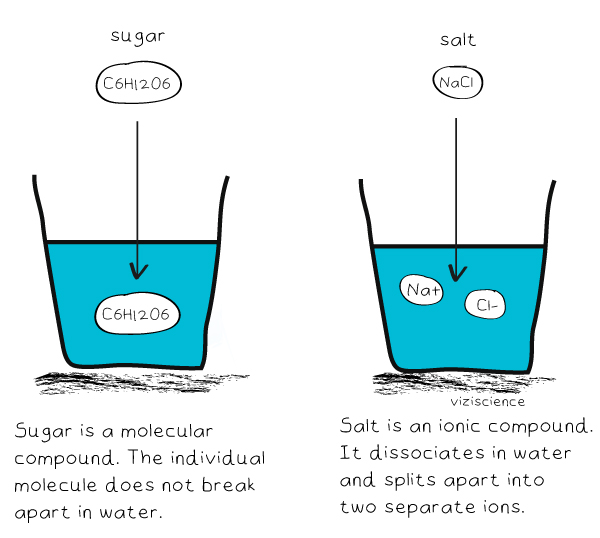
Salt Vs Sugar Chemistry . Instead, they share their electrons. Table sugar or sucrose differs from salt in the bonding between its atoms. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: While sugar and salt may look similar, they are quite different. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. The atoms in sugar do not form ions; Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Zoom in to see how different sugar and salt. What happens when sugar and salt are added to water?
from www.pro-factory-plus.eu
The atoms in sugar do not form ions; While sugar and salt may look similar, they are quite different. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: What happens when sugar and salt are added to water? Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Instead, they share their electrons. Table sugar or sucrose differs from salt in the bonding between its atoms. Zoom in to see how different sugar and salt.
Molecular Formula Of Sugar Water ProFactoryPlus Perspective
Salt Vs Sugar Chemistry The atoms in sugar do not form ions; Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: While sugar and salt may look similar, they are quite different. Instead, they share their electrons. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. Table sugar or sucrose differs from salt in the bonding between its atoms. Zoom in to see how different sugar and salt. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. What happens when sugar and salt are added to water? The atoms in sugar do not form ions;
From sciencenotes.org
How to Separate Salt and Sugar Salt Vs Sugar Chemistry While sugar and salt may look similar, they are quite different. The atoms in sugar do not form ions; What happens when sugar and salt are added to water? Table sugar or sucrose differs from salt in the bonding between its atoms. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Sugar. Salt Vs Sugar Chemistry.
From www.youtube.com
Salt vs Sugar Test 2 YouTube Salt Vs Sugar Chemistry Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Instead, they share their electrons. While sugar and salt may look similar, they are quite different. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties. Salt Vs Sugar Chemistry.
From www.youtube.com
Sugar vs Salt Under Microscope YouTube Salt Vs Sugar Chemistry The atoms in sugar do not form ions; This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. What happens when sugar and salt are added to. Salt Vs Sugar Chemistry.
From thecontentauthority.com
Salt vs Sugar Deciding Between Similar Terms Salt Vs Sugar Chemistry What happens when sugar and salt are added to water? Table sugar or sucrose differs from salt in the bonding between its atoms. Instead, they share their electrons. Zoom in to see how different sugar and salt. While sugar and salt may look similar, they are quite different. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements. Salt Vs Sugar Chemistry.
From gorphysanon.medium.com
Salt Vs Sugar. Salt and Sugar are the crystals that… by Gorphy Sanon Medium Salt Vs Sugar Chemistry Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: While sugar and salt may look similar, they are quite different. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. What happens when sugar and salt are added to water? Instead, they share their electrons. Pour. Salt Vs Sugar Chemistry.
From www.scribd.com
effect on table salt vs table sugar on the boiling point water Sodium Chloride Water Salt Vs Sugar Chemistry Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. Instead, they share their electrons. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve. Salt Vs Sugar Chemistry.
From www.madebyteachers.com
Solubility of Powders in Water Activity Hands on Science Made By Teachers Salt Vs Sugar Chemistry Zoom in to see how different sugar and salt. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose,. Salt Vs Sugar Chemistry.
From www.youtube.com
Salt vs. Sugar Which Dissolves Faster YouTube Salt Vs Sugar Chemistry The atoms in sugar do not form ions; Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties. Salt Vs Sugar Chemistry.
From calories-info.com
Salt vs Sugar What Should You Choose? Salt Vs Sugar Chemistry Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. What happens when sugar and salt are added to water? The atoms in sugar do not form ions; Instead, they share their electrons. Sugar. Salt Vs Sugar Chemistry.
From sciencenotes.org
How to Separate Salt and Sugar Salt Vs Sugar Chemistry What happens when sugar and salt are added to water? The atoms in sugar do not form ions; This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Table sugar or sucrose differs from salt in the bonding between its atoms. Various sugars are naturally occurring,. Salt Vs Sugar Chemistry.
From www.chegg.com
Solved [5 Points] 4. With reference to the solubility of Salt Vs Sugar Chemistry Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Instead, they share their electrons. What happens when sugar and salt are added to water? This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Sugar has the chemical. Salt Vs Sugar Chemistry.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Salt Vs Sugar Chemistry Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Table sugar or sucrose differs from salt in the bonding between its atoms. While sugar and salt may look similar, they are. Salt Vs Sugar Chemistry.
From www.pinterest.com
Salt & Sugar Taste Test Five Senses Activity Modern Homestead Mama Lembar kerja, Kerja Salt Vs Sugar Chemistry Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Zoom in to see how. Salt Vs Sugar Chemistry.
From organicelementsspa.com
The Pros and Cons Of Salt vs Sugar Scrubs Organic Elements Spa Salt Vs Sugar Chemistry While sugar and salt may look similar, they are quite different. Table sugar or sucrose differs from salt in the bonding between its atoms. Instead, they share their electrons. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: The atoms in sugar do not form ions; What happens when sugar and salt are added to. Salt Vs Sugar Chemistry.
From www.youtube.com
Sucrose/Sugar vs Sodium Chloride/Table Salt (Solubility on Temperature Chemistry Experiment Salt Vs Sugar Chemistry Zoom in to see how different sugar and salt. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. The atoms in sugar do not form ions; Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: What happens when sugar and. Salt Vs Sugar Chemistry.
From www.reddit.com
4 Feb 7, 2021 KidCurios Salt Vs Sugar Chemistry What happens when sugar and salt are added to water? Zoom in to see how different sugar and salt. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water.. Salt Vs Sugar Chemistry.
From www.pro-factory-plus.eu
Molecular Formula Of Sugar Water ProFactoryPlus Perspective Salt Vs Sugar Chemistry Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. Zoom in to see how different sugar and salt. Instead, they share their electrons. What happens when sugar and salt are added to water? Sugar has the. Salt Vs Sugar Chemistry.
From www.madebyteachers.com
Solubility of Powders in Water Activity Hands on Science Made By Teachers Salt Vs Sugar Chemistry Instead, they share their electrons. Table sugar or sucrose differs from salt in the bonding between its atoms. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: What happens when sugar and salt are added to water? The. Salt Vs Sugar Chemistry.
From foodstruct.com
Sugar substitute vs. Table salt — InDepth Nutrition Comparison Salt Vs Sugar Chemistry This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. The atoms in sugar do not form ions; Table sugar or sucrose differs from salt in the. Salt Vs Sugar Chemistry.
From elchoroukhost.net
Table Salt Chemical Compound Formula Elcho Table Salt Vs Sugar Chemistry This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Zoom in to see how different sugar and salt. Table sugar or sucrose differs from salt in the bonding between its atoms. Pour in sugar, shake in salt, and evaporate water to see the effects on. Salt Vs Sugar Chemistry.
From www.slideserve.com
PPT Regents Chemistry PowerPoint Presentation, free download ID861994 Salt Vs Sugar Chemistry What happens when sugar and salt are added to water? Instead, they share their electrons. While sugar and salt may look similar, they are quite different. Table sugar or sucrose differs from salt in the bonding between its atoms. Zoom in to see how different sugar and salt. This formative assessment looks at two household chemicals (table salt and sugar). Salt Vs Sugar Chemistry.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Salt Vs Sugar Chemistry What happens when sugar and salt are added to water? Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Zoom in to see how different sugar and salt. Instead, they share. Salt Vs Sugar Chemistry.
From www.chicagovitality.com
Sugar vs. Salt which is worse? Chicago Vitality Men's Health Salt Vs Sugar Chemistry This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Zoom in to see how different sugar and salt. While sugar and salt may look similar, they are quite different. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Sugar has. Salt Vs Sugar Chemistry.
From www.dreamstime.com
Ionic Versus Covalent Compounds in Solution Stock Vector Illustration of molecular, crystal Salt Vs Sugar Chemistry Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. The atoms in sugar do not form ions; What happens when sugar and salt are added to water? Sugar. Salt Vs Sugar Chemistry.
From foodstruct.com
Sugar vs. Table salt — InDepth Nutrition Comparison Salt Vs Sugar Chemistry While sugar and salt may look similar, they are quite different. The atoms in sugar do not form ions; Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. Table sugar or sucrose differs from salt in the bonding between its atoms. Pour in sugar, shake in salt, and evaporate water. Salt Vs Sugar Chemistry.
From www.numerade.com
SOLVED Show equation(s) for the dissolution of salt vs. sugar to explain (at a molecular level Salt Vs Sugar Chemistry The atoms in sugar do not form ions; Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Table sugar or sucrose differs from salt. Salt Vs Sugar Chemistry.
From www.youtube.com
Solubility Lab Salt vs. Sugar YouTube Salt Vs Sugar Chemistry Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water.. Salt Vs Sugar Chemistry.
From www.visionlearning.com
Properties of Solids Chemistry Visionlearning Salt Vs Sugar Chemistry Zoom in to see how different sugar and salt. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. Table sugar or sucrose differs from salt in the bonding between its atoms. While sugar and salt may look similar, they are quite different. Sugar has the. Salt Vs Sugar Chemistry.
From irvin-bogspotlevy.blogspot.com
Are Table Sugar and Table Salt Pure Substances Salt Vs Sugar Chemistry This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. The atoms in sugar do not form ions; Zoom in to see how different sugar and salt. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of.. Salt Vs Sugar Chemistry.
From www.youtube.com
How to Make Salt or Sugar Water Density Rainbow Tower Simple Kids Science YouTube Salt Vs Sugar Chemistry Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Zoom in to see how different sugar and salt. The atoms in sugar do not form ions; Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from. Salt Vs Sugar Chemistry.
From www.youtube.com
Solutes in Water (Salt vs Sugar) YouTube Salt Vs Sugar Chemistry Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Zoom in to see how different sugar and salt. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. Table sugar or sucrose differs from salt in the bonding between its atoms. Instead, they share their electrons.. Salt Vs Sugar Chemistry.
From www.baking-sense.com
The Science of Salt as a Baking Ingredient Baking Sense® Salt Vs Sugar Chemistry Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: The atoms in sugar do not form ions; Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Table sugar or sucrose differs from salt in the bonding between its atoms. Zoom in to see how different sugar and. Salt Vs Sugar Chemistry.
From www.chemedx.org
Salt vs. Sugar A Dissolving Problem Chemical Education Xchange Salt Vs Sugar Chemistry Zoom in to see how different sugar and salt. Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. This formative assessment looks at two household chemicals (table salt and sugar) and compares their properties while looking at how they dissolve in water. What happens when sugar and salt are added. Salt Vs Sugar Chemistry.
From www.visionlearning.org
Properties of Solids Chemistry Visionlearning Salt Vs Sugar Chemistry Various sugars are naturally occurring, but the term “sugar” usually refers to sucrose, which is a disaccharide made of. While sugar and salt may look similar, they are quite different. Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Instead, they share their electrons. The atoms in sugar do not form ions;. Salt Vs Sugar Chemistry.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Salt Vs Sugar Chemistry Zoom in to see how different sugar and salt. Table sugar or sucrose differs from salt in the bonding between its atoms. Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different elements than salt: Instead, they share their electrons. The atoms in sugar do not form ions; Sugar has the chemical formulate \(\ce{c12h22o11}\) and is constructed from different. Salt Vs Sugar Chemistry.