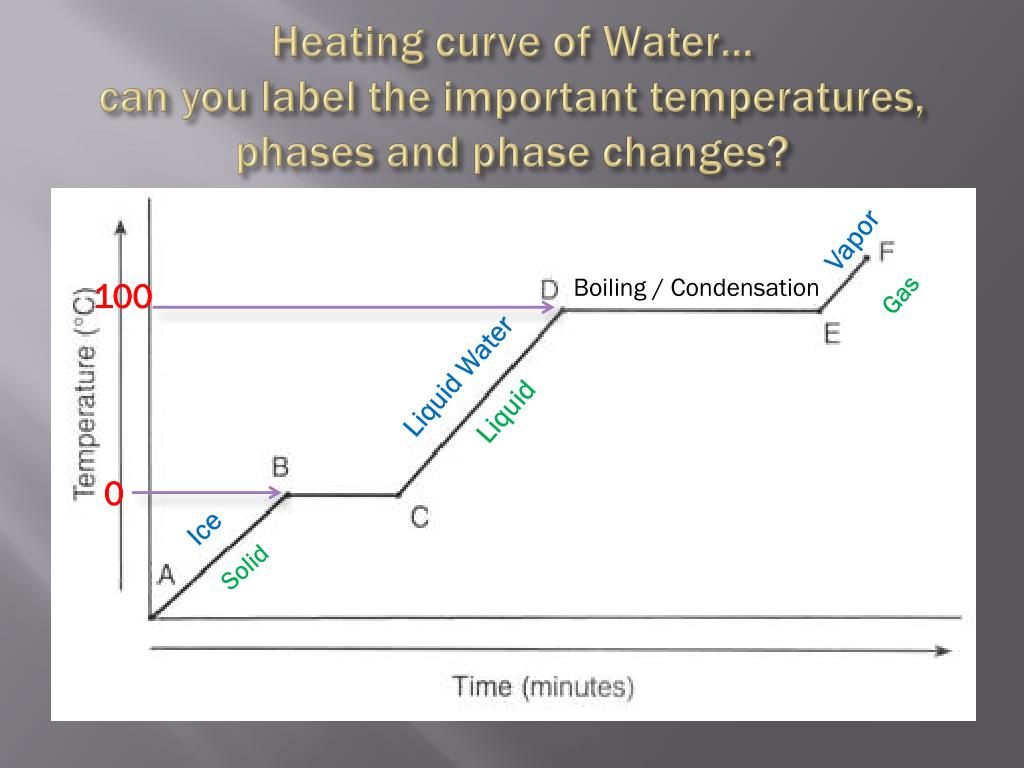
Heating Curve Of Ice . To calculate the energy changes that accompany phase changes. As heat is steadily added to the ice block, the water molecules will begin to. The slope of the line depends on how quickly we added heat. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. The sample is initially ice at 1 atm and. The melting point of ice is 0oc. As heat is added to ice, the temperature begins to increase. Q = m × c × δ t (see previous chapter on. The ice is in a closed container. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The heat needed to change the temperature of a given substance (with no change in phase) is: We take advantage of changes between the gas, liquid, and solid states. Heat is flowing at a constant rate, thus time can be interpreted as heat. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The temperature of the system is plotted as function of time.
from www.slideserve.com
The slope of the line depends on how quickly we added heat. Q = m × c × δ t (see previous chapter on. The ice is in a closed container. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. We take advantage of changes between the gas, liquid, and solid states. If our stove is on a high setting, the graph's. The sample is initially ice at 1 atm and. Heat is flowing at a constant rate, thus time can be interpreted as heat. As heat is added to ice, the temperature begins to increase. The heat needed to change the temperature of a given substance (with no change in phase) is:
PPT Heating Curves and Thermodynamics PowerPoint Presentation, free
Heating Curve Of Ice The ice is in a closed container. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. As heat is steadily added to the ice block, the water molecules will begin to. If our stove is on a high setting, the graph's. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heat is flowing at a constant rate, thus time can be interpreted as heat. The heat needed to change the temperature of a given substance (with no change in phase) is: The sample is initially ice at 1 atm and. As heat is added to ice, the temperature begins to increase. The temperature of the system is plotted as function of time. The ice is in a closed container. The melting point of ice is 0oc. The slope of the line depends on how quickly we added heat. To calculate the energy changes that accompany phase changes. We take advantage of changes between the gas, liquid, and solid states.
From www.showme.com
Heating Curves Science, Chemistry ShowMe Heating Curve Of Ice As heat is steadily added to the ice block, the water molecules will begin to. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. If our stove is on a high setting, the graph's. To calculate the energy changes that accompany phase changes. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases.. Heating Curve Of Ice.
From www.slideserve.com
PPT Heating Curves and Thermodynamics PowerPoint Presentation, free Heating Curve Of Ice To calculate the energy changes that accompany phase changes. Q = m × c × δ t (see previous chapter on. The temperature of the system is plotted as function of time. As heat is added to ice, the temperature begins to increase. If our stove is on a high setting, the graph's. The arrows trace a path of increasing. Heating Curve Of Ice.
From byjus.com
The heating curve for ice initially at a particular pressure is given Heating Curve Of Ice We take advantage of changes between the gas, liquid, and solid states. Heat is flowing at a constant rate, thus time can be interpreted as heat. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The heat needed to change the temperature of a given substance (with no change in phase) is: The melting point of ice is 0oc. Q = m ×. Heating Curve Of Ice.
From lessonlibnurselings.z21.web.core.windows.net
Heating And Cooling Curve Explanation Heating Curve Of Ice The temperature of the system is plotted as function of time. To calculate the energy changes that accompany phase changes. We take advantage of changes between the gas, liquid, and solid states. If our stove is on a high setting, the graph's. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample. Heating Curve Of Ice.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes (Problems) Chemistry LibreTexts Heating Curve Of Ice The heat needed to change the temperature of a given substance (with no change in phase) is: The slope of the line depends on how quickly we added heat. The melting point of ice is 0oc. The temperature of the system is plotted as function of time. We take advantage of changes between the gas, liquid, and solid states. The. Heating Curve Of Ice.
From www.youtube.com
CALORIMETRY Heating Curve of ICE (PHASE CHANGES GRAPH ) PPV Part 2 Heating Curve Of Ice Q = m × c × δ t (see previous chapter on. The slope of the line depends on how quickly we added heat. Heat is flowing at a constant rate, thus time can be interpreted as heat. The temperature of the system is plotted as function of time. To calculate the energy changes that accompany phase changes. The arrows. Heating Curve Of Ice.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Heating Curve Of Ice The sample is initially ice at 1 atm and. The temperature of the system is plotted as function of time. As heat is added to ice, the temperature begins to increase. We take advantage of changes between the gas, liquid, and solid states. The slope of the line depends on how quickly we added heat. If our stove is on. Heating Curve Of Ice.
From chem-net.blogspot.com
Phase Changes Energy Changes Heating Curves Chemistry Net Heating Curve Of Ice To calculate the energy changes that accompany phase changes. The heat needed to change the temperature of a given substance (with no change in phase) is: Q = m × c × δ t (see previous chapter on. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. The. Heating Curve Of Ice.
From www.youtube.com
AP Video 10.6 Intro to HeatingCooling Curves & Calculations YouTube Heating Curve Of Ice The sample is initially ice at 1 atm and. The heat needed to change the temperature of a given substance (with no change in phase) is: Heat is flowing at a constant rate, thus time can be interpreted as heat. If our stove is on a high setting, the graph's. The arrows trace a path of increasing temperature at a. Heating Curve Of Ice.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Heating Curve Of Ice As heat is steadily added to the ice block, the water molecules will begin to. Heat is flowing at a constant rate, thus time can be interpreted as heat. The ice is in a closed container. The slope of the line depends on how quickly we added heat. We take advantage of changes between the gas, liquid, and solid states.. Heating Curve Of Ice.
From www.chegg.com
Solved The figure shown below is the heating curve obtained Heating Curve Of Ice We take advantage of changes between the gas, liquid, and solid states. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The sample is initially ice at 1 atm and. If our stove is on a high setting, the graph's. Q = m. Heating Curve Of Ice.
From solvedlib.com
Draw a heating curve for a sample of ice that is heat… SolvedLib Heating Curve Of Ice The melting point of ice is 0oc. As heat is steadily added to the ice block, the water molecules will begin to. Heat is flowing at a constant rate, thus time can be interpreted as heat. If our stove is on a high setting, the graph's. The ice is in a closed container. The sample is initially ice at 1. Heating Curve Of Ice.
From www.showme.com
Heating and Cooling Curves Explained Science, Heating Curve, Cooling Heating Curve Of Ice Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The temperature of the system is plotted as function of time. To calculate the energy changes that accompany phase changes. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. The slope of the line depends on how quickly we added heat. The. Heating Curve Of Ice.
From www.owhentheyanks.com
Heating And Cooling Curve Worksheet Heating Curve Of Ice The sample is initially ice at 1 atm and. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. As heat is added to ice, the temperature begins to increase. The temperature of the system is plotted as function of time. The heat needed to change the temperature of a given substance (with no change in phase) is: To calculate the energy changes that. Heating Curve Of Ice.
From circuitdiagramalexandra.z5.web.core.windows.net
Heating Curve Diagram Heating Curve Of Ice Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. Q = m × c × δ t (see previous chapter on. The. Heating Curve Of Ice.
From joiwdmstd.blob.core.windows.net
Heating Ice Graph at Herbert Teems blog Heating Curve Of Ice Q = m × c × δ t (see previous chapter on. As heat is added to ice, the temperature begins to increase. If our stove is on a high setting, the graph's. The slope of the line depends on how quickly we added heat. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. Heat is flowing at a constant rate, thus time. Heating Curve Of Ice.
From scienceisntscary.wordpress.com
Image Heating Curve Of Ice Heat is flowing at a constant rate, thus time can be interpreted as heat. The melting point of ice is 0oc. The sample is initially ice at 1 atm and. The ice is in a closed container. As heat is added to ice, the temperature begins to increase. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. If our stove is on a. Heating Curve Of Ice.
From printableroparstvaoy.z4.web.core.windows.net
Heating Curve Of Water Explained Heating Curve Of Ice Heat is flowing at a constant rate, thus time can be interpreted as heat. The temperature of the system is plotted as function of time. As heat is added to ice, the temperature begins to increase. To calculate the energy changes that accompany phase changes. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a. Heating Curve Of Ice.
From www.youtube.com
CALORIMETRY Heating Curve of ICE (3D animation) YouTube Heating Curve Of Ice We take advantage of changes between the gas, liquid, and solid states. The melting point of ice is 0oc. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The ice is in a closed container. As heat is added to ice, the temperature begins to increase. Property get [map. Heating Curve Of Ice.
From www.numerade.com
SOLVED This diagram shows a heating curve for ice beginning at 25 °C Heating Curve Of Ice If our stove is on a high setting, the graph's. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The slope of the line depends on how quickly we added heat. Q = m × c × δ t (see previous chapter on. As heat is added to ice, the temperature begins to increase. The ice is in a closed container. To calculate. Heating Curve Of Ice.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve Of Ice As heat is steadily added to the ice block, the water molecules will begin to. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. The temperature of the system is plotted as function of time. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The sample is initially ice at 1. Heating Curve Of Ice.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curve Of Ice The heat needed to change the temperature of a given substance (with no change in phase) is: The sample is initially ice at 1 atm and. The melting point of ice is 0oc. Heat is flowing at a constant rate, thus time can be interpreted as heat. As heat is added to ice, the temperature begins to increase. The arrows. Heating Curve Of Ice.
From www.slideserve.com
PPT Matter Properties and Changes PowerPoint Presentation, free Heating Curve Of Ice The sample is initially ice at 1 atm and. Q = m × c × δ t (see previous chapter on. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The ice is in a closed container. As heat is added to ice, the temperature begins to increase. The. Heating Curve Of Ice.
From brainly.in
To study the effect of heat on ice by using a graph Brainly.in Heating Curve Of Ice Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Q = m × c × δ t (see previous chapter on. If our stove is on a high setting, the graph's. The heat needed to change the temperature of a given substance (with no change in phase) is: The. Heating Curve Of Ice.
From www.youtube.com
Time lapse heating curve of ice below its freezing point YouTube Heating Curve Of Ice As heat is steadily added to the ice block, the water molecules will begin to. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The slope of the line depends on how quickly we added heat. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. The ice is in a closed. Heating Curve Of Ice.
From study.com
What are Heating and Cooling Curves? Video & Lesson Transcript Heating Curve Of Ice Q = m × c × δ t (see previous chapter on. To calculate the energy changes that accompany phase changes. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. If our stove is on a high setting, the graph's. We take. Heating Curve Of Ice.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts Heating Curve Of Ice The temperature of the system is plotted as function of time. The ice is in a closed container. The heat needed to change the temperature of a given substance (with no change in phase) is: The slope of the line depends on how quickly we added heat. The sample is initially ice at 1 atm and. As heat is added. Heating Curve Of Ice.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Of Ice As heat is added to ice, the temperature begins to increase. Heat is flowing at a constant rate, thus time can be interpreted as heat. As heat is steadily added to the ice block, the water molecules will begin to. If our stove is on a high setting, the graph's. The heat needed to change the temperature of a given. Heating Curve Of Ice.
From joiwdmstd.blob.core.windows.net
Heating Ice Graph at Herbert Teems blog Heating Curve Of Ice Heat is flowing at a constant rate, thus time can be interpreted as heat. As heat is added to ice, the temperature begins to increase. The slope of the line depends on how quickly we added heat. The melting point of ice is 0oc. The sample is initially ice at 1 atm and. Figure 11.4.1 shows a heating curve, a. Heating Curve Of Ice.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Heating Curve Of Ice The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. The melting point of ice is 0oc. As heat is steadily added to the ice block, the water molecules will begin to. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g. Heating Curve Of Ice.
From mavink.com
What Is A Heating Curve Heating Curve Of Ice We take advantage of changes between the gas, liquid, and solid states. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. Property get [map mindtouch.deki.logic.extensionprocessorqueryprovider+<>c__displayclass230_0.b__1] (), 11.02:_solids_liquids_and_gases. The melting point of ice is 0oc. The slope of the line depends on how quickly we added heat. The temperature. Heating Curve Of Ice.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curve Of Ice If our stove is on a high setting, the graph's. The melting point of ice is 0oc. We take advantage of changes between the gas, liquid, and solid states. The ice is in a closed container. The slope of the line depends on how quickly we added heat. The heat needed to change the temperature of a given substance (with. Heating Curve Of Ice.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID4251370 Heating Curve Of Ice As heat is steadily added to the ice block, the water molecules will begin to. Heat is flowing at a constant rate, thus time can be interpreted as heat. The sample is initially ice at 1 atm and. The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. The. Heating Curve Of Ice.
From studyschoolburman.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating Curve Of Ice The arrows trace a path of increasing temperature at a constant pressure, the same pressure as used in the heating curve. The temperature of the system is plotted as function of time. If our stove is on a high setting, the graph's. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample. Heating Curve Of Ice.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating Curve Of Ice The slope of the line depends on how quickly we added heat. To calculate the energy changes that accompany phase changes. The heat needed to change the temperature of a given substance (with no change in phase) is: The melting point of ice is 0oc. The sample is initially ice at 1 atm and. Heat is flowing at a constant. Heating Curve Of Ice.