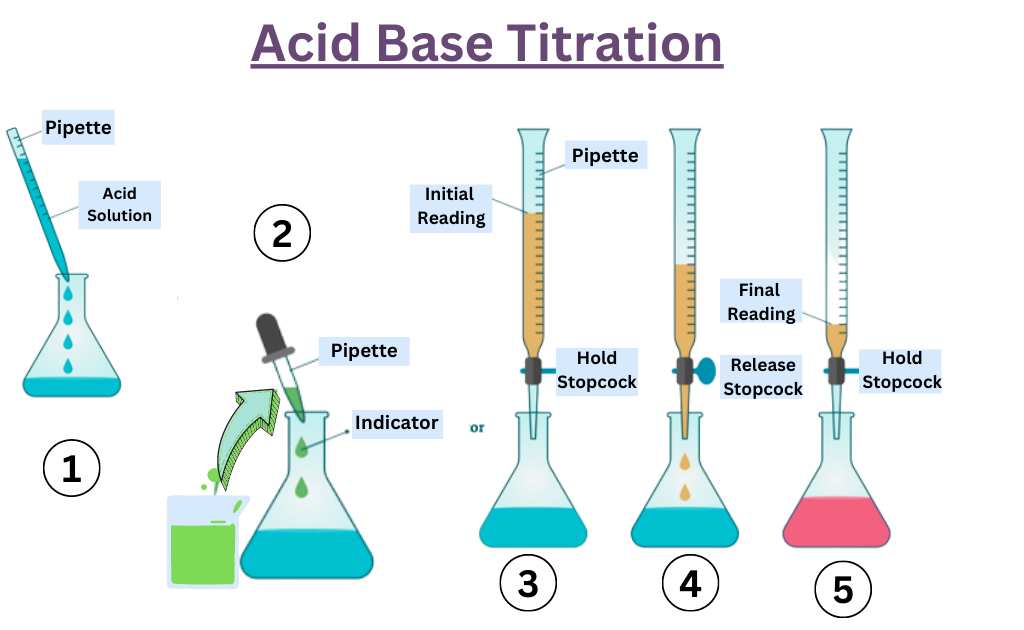
Titration And Its Types Pdf . Titration is a quantitative technique that uses a solution of known concentration to react completely with an. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred.
from themasterchemistry.com
Titration is a quantitative technique that uses a solution of known concentration to react completely with an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always.
Acid Base TitrationWorking Principle, Process, Types And Indicators
Titration And Its Types Pdf A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always.
From www.studypool.com
SOLUTION Titration and its types Studypool Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is the process of determining the quantity of a substance a by adding. Titration And Its Types Pdf.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. A titration is the process of. Titration And Its Types Pdf.
From www.studypool.com
SOLUTION Titration and its types Studypool Titration And Its Types Pdf Titration is a quantitative technique that uses a solution of known concentration to react completely with an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred.. Titration And Its Types Pdf.
From www.scribd.com
AP Titrations Presentation PDF Chemistry Titration Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. Titration is a quantitative technique that uses a solution of known concentration to react completely with an.. Titration And Its Types Pdf.
From www.studypool.com
SOLUTION Titration and its types Studypool Titration And Its Types Pdf A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;.. Titration And Its Types Pdf.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Titration And Its Types Pdf A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is. Titration And Its Types Pdf.
From www.scribd.com
typesoftitration.pdf Titration Chemistry Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. Titration is a quantitative technique that uses a solution of known concentration to react completely. Titration And Its Types Pdf.
From letitsnowglobe.co.uk
Titration procedure pdf Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. Titration is a quantitative technique that uses a solution of known concentration to react completely with an.. Titration And Its Types Pdf.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration And Its Types Pdf Titration is a quantitative technique that uses a solution of known concentration to react completely with an. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent. Titration And Its Types Pdf.
From www.academia.edu
(PDF) Basics of titration Professor Dr. Loutfy H . Madkour Academia.edu Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. A titration is a quantitative,. Titration And Its Types Pdf.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Titration And Its Types Pdf A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;.. Titration And Its Types Pdf.
From www.youtube.com
TYPES OF TITRATION IN CHEMISTRY ACID BASE TITRATION REDOX TITRATION Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always. Titration is a quantitative technique that uses a. Titration And Its Types Pdf.
From exyyylzwb.blob.core.windows.net
Titration In Pharmaceutical Industry at Jamel Edwards blog Titration And Its Types Pdf A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how. Titration And Its Types Pdf.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A titration is the process of determining the quantity of a substance a by adding. Titration And Its Types Pdf.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. Titration is a quantitative technique that uses a solution of known concentration to react completely. Titration And Its Types Pdf.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine. Titration And Its Types Pdf.
From www.studypool.com
SOLUTION Titration and its types Studypool Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. 1.1 a titration involves measuring. Titration And Its Types Pdf.
From letitsnowglobe.co.uk
Titration procedure pdf Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. 1.1 a titration involves measuring the. Titration And Its Types Pdf.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a. Titration And Its Types Pdf.
From www.studypool.com
SOLUTION Titration and its types Studypool Titration And Its Types Pdf Titration is a quantitative technique that uses a solution of known concentration to react completely with an. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. Understand. Titration And Its Types Pdf.
From mavink.com
Titration Picture Titration And Its Types Pdf Titration is a quantitative technique that uses a solution of known concentration to react completely with an. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which. Titration And Its Types Pdf.
From www.studypool.com
SOLUTION Titration and its types Studypool Titration And Its Types Pdf A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction. Titration And Its Types Pdf.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always. Titration is a quantitative technique that uses a. Titration And Its Types Pdf.
From www.studypool.com
SOLUTION Titrations and types Studypool Titration And Its Types Pdf A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which. Titration And Its Types Pdf.
From joioqtatt.blob.core.windows.net
Types Of Titration Reactions at Alex Ortiz blog Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which. Titration And Its Types Pdf.
From loeppodiz.blob.core.windows.net
Titration Calculation Step By Step Pdf at Linda Tibbetts blog Titration And Its Types Pdf A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. 1.1 a titration involves measuring the exact volume. Titration And Its Types Pdf.
From www.scribd.com
About Titration and Types PDF Chemistry Titration Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. A titration is a quantitative,. Titration And Its Types Pdf.
From collegedunia.com
Titration Formula Types, Process & Acid Base Indicators Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration. Titration And Its Types Pdf.
From www.scribd.com
Types of Titrations PDF Iodine Titration Titration And Its Types Pdf A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always. Titration is a quantitative technique that uses a solution of known concentration to react completely with. Titration And Its Types Pdf.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration And Its Types Pdf A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it reacts (almost always. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is. Titration And Its Types Pdf.
From www.scribd.com
Titration (Chemistry Experiment Report) PDF Titration Chemistry Titration And Its Types Pdf A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred.. Titration And Its Types Pdf.
From www.scribd.com
Titration PDF Titration Chemistry Titration And Its Types Pdf Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent. Titration And Its Types Pdf.
From theedge.com.hk
Chemistry How To Titration The Edge Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. Titration is a quantitative technique that uses a solution of known concentration to react completely with an. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred.. Titration And Its Types Pdf.
From slidetodoc.com
Chapter 15 Redox Titrations 15 1 The Shape Titration And Its Types Pdf 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another reagent in solution;. A procedure in which one substance (titrant) is carefully added to another (analyte) until complete reaction has occurred. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an.. Titration And Its Types Pdf.
From byjus.com
What is Titration? What are its types? Explain briefly Titration And Its Types Pdf Titration is a quantitative technique that uses a solution of known concentration to react completely with an. A titration is a quantitative, volumetric procedure used in analytical chemistry to determine the concentration of an. Understand and apply the equilibrium constant based on concentration of species (kc) to predict how far a reaction will proceed. A titration is the process of. Titration And Its Types Pdf.