Heat Capacity Entropy Equation . the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. Consider adding a fixed amount of heat to a. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. heat capacities in enthalpy and entropy calculations. Isothermal steps have a change in. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses.
from www.slideserve.com
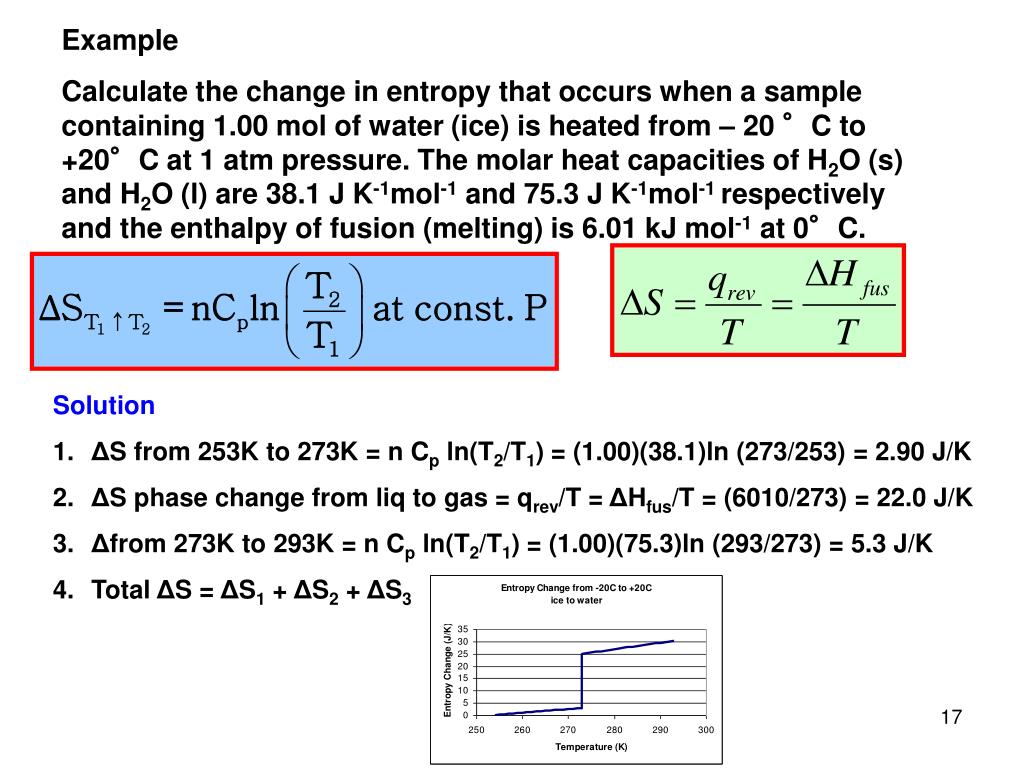
heat capacities in enthalpy and entropy calculations. Consider adding a fixed amount of heat to a. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Isothermal steps have a change in. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal.
PPT Chapter 15 Spontaneity, Entropy, and Free Energy PowerPoint
Heat Capacity Entropy Equation learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. heat capacities in enthalpy and entropy calculations. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Isothermal steps have a change in. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. Consider adding a fixed amount of heat to a.
From studylibrarygodward.z13.web.core.windows.net
Equation To Calculate Specific Heat Heat Capacity Entropy Equation Consider adding a fixed amount of heat to a. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. learn how to calculate and interpret heat capacity, enthalpy, and. Heat Capacity Entropy Equation.
From www.youtube.com
REFERENCE ENTROPY and Specific Heats in 12 Minutes! YouTube Heat Capacity Entropy Equation the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Isothermal steps have a change in. the second law of thermodynamics states that the total entropy. Heat Capacity Entropy Equation.
From physics.stackexchange.com
thermodynamics Formula of Entropy Physics Stack Exchange Heat Capacity Entropy Equation learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances. Heat Capacity Entropy Equation.
From www.youtube.com
Change in ENTHALPY Using Specific Heat at Constant Pressure in 3 Heat Capacity Entropy Equation Consider adding a fixed amount of heat to a. Isothermal steps have a change in. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. . Heat Capacity Entropy Equation.
From studylib.net
Entropy Change by Heat Transfer Heat Capacity Entropy Equation learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Consider adding a fixed amount of heat to a. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. the heat flow is calculated from the first law of thermodynamics, q = δeint. Heat Capacity Entropy Equation.
From www.slideserve.com
PPT Thermodynamics I Chapter 2 Properties of Pure Substances Heat Capacity Entropy Equation the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric,. Heat Capacity Entropy Equation.
From www.slideserve.com
PPT Chapter 15 Spontaneity, Entropy, and Free Energy PowerPoint Heat Capacity Entropy Equation the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. heat capacities in enthalpy and entropy calculations. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. Consider adding a fixed. Heat Capacity Entropy Equation.
From enterpriseshery.weebly.com
How to calculate entropy change thermodynamics enterpriseshery Heat Capacity Entropy Equation Isothermal steps have a change in. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. Consider adding a fixed amount of heat to a. learn how to calculate. Heat Capacity Entropy Equation.
From www.slideserve.com
PPT Chapter 17 The first law of thermodynamics PowerPoint Heat Capacity Entropy Equation Consider adding a fixed amount of heat to a. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. Isothermal steps have a change in. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. learn. Heat Capacity Entropy Equation.
From www.slideserve.com
PPT Find the change in entropy when 87.3 g of water vapor condense Heat Capacity Entropy Equation learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in. Heat Capacity Entropy Equation.
From www.youtube.com
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi Heat Capacity Entropy Equation heat capacities in enthalpy and entropy calculations. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. the heat capacity is the slope of. Heat Capacity Entropy Equation.
From www.youtube.com
Enthalpy and Specific Heat Derivation Cp = Cv + R YouTube Heat Capacity Entropy Equation the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. measurements of the heat capacity of a substance and the enthalpies of. Heat Capacity Entropy Equation.
From www.youtube.com
Heat capacity Thermodynamics AP Chemistry Khan Academy YouTube Heat Capacity Entropy Equation the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Consider adding a fixed amount of heat to a. heat capacities in enthalpy and entropy calculations.. Heat Capacity Entropy Equation.
From studylib.net
Heat Equation Heat Capacity Entropy Equation learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Isothermal steps have a change in. Consider adding a fixed amount of heat to a. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. the. Heat Capacity Entropy Equation.
From slideplayer.com
Now we can write And or Taking S = S(U,V) we can also write By Heat Capacity Entropy Equation Consider adding a fixed amount of heat to a. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. the heat flow is calculated from the first law of. Heat Capacity Entropy Equation.
From www.engineeringstream.com
LAW OF THERMODYNAMICS Engineering Stream Heat Capacity Entropy Equation learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. the heat flow is calculated from the first law of thermodynamics, q = δeint −. Heat Capacity Entropy Equation.
From www.numerade.com
SOLVED The heat capacity at constant pressure (Cp) is the partial Heat Capacity Entropy Equation learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint =. Heat Capacity Entropy Equation.
From www.youtube.com
Specific Heat capacities of gas Cp and Cv First law of Heat Capacity Entropy Equation heat capacities in enthalpy and entropy calculations. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Consider adding a fixed amount of heat to a. Isothermal steps have a change in. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used. Heat Capacity Entropy Equation.
From slidetodoc.com
Sensible Heat and Enthalpy Calculations constant A Enthalpy Heat Capacity Entropy Equation the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. heat capacities in enthalpy and entropy calculations. Isothermal steps have a change in. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Consider adding a fixed amount of. Heat Capacity Entropy Equation.
From www.youtube.com
Entropy By Heat Flow YouTube Heat Capacity Entropy Equation measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. Isothermal steps have a change in. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. heat capacities in enthalpy and. Heat Capacity Entropy Equation.
From www.youtube.com
Calculating Enthalpy and Entropy Using the NIST Book YouTube Heat Capacity Entropy Equation Consider adding a fixed amount of heat to a. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. learn how to calculate and interpret. Heat Capacity Entropy Equation.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 10, pt 1 of 2 Entropy Heat Capacity Entropy Equation learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to. Heat Capacity Entropy Equation.
From www.linkedin.com
Is specific heat the same as entropy? Why both have the same SI unit kJ Heat Capacity Entropy Equation Consider adding a fixed amount of heat to a. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. heat capacities in enthalpy and entropy calculations. learn how. Heat Capacity Entropy Equation.
From www.youtube.com
3.2 Entropy and Heat (Thermal Physics) (Schroeder) YouTube Heat Capacity Entropy Equation the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. Consider adding a fixed amount of heat to a. learn how to. Heat Capacity Entropy Equation.
From www.researchgate.net
(a) Enthalpy, entropy, and free energy (b) heat capacity for perfect Heat Capacity Entropy Equation the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. Isothermal steps have a change in. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for. Heat Capacity Entropy Equation.
From elife-news.blogspot.com
ELifes Exercise Thermodynamics (enthalpy, heat of melting, heat Heat Capacity Entropy Equation measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. heat capacities in enthalpy and entropy calculations. the heat flow. Heat Capacity Entropy Equation.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Heat Capacity Entropy Equation learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. heat capacities in enthalpy and entropy calculations. the second law of thermodynamics states that the total entropy of a system either increases. Heat Capacity Entropy Equation.
From www.grc.nasa.gov
Enthalpy Heat Capacity Entropy Equation the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data. Heat Capacity Entropy Equation.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3150253 Heat Capacity Entropy Equation Consider adding a fixed amount of heat to a. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. heat capacities in enthalpy and entropy calculations. Isothermal steps have a change in. measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used. Heat Capacity Entropy Equation.
From physics.stackexchange.com
thermodynamics Derivation of heat capacity at constant pressure and Heat Capacity Entropy Equation heat capacities in enthalpy and entropy calculations. Isothermal steps have a change in. learn how to calculate entropy changes for different types of processes, such as isothermal, isobaric, isochoric, and. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. the heat capacity is the. Heat Capacity Entropy Equation.
From www.grc.nasa.gov
Specific Heats Heat Capacity Entropy Equation learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Isothermal steps have a change in. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. learn how to calculate entropy changes for different types of. Heat Capacity Entropy Equation.
From www.youtube.com
Ratio Of Heat Capacities Thermodynamic Relations Engineering Heat Capacity Entropy Equation Consider adding a fixed amount of heat to a. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. the heat capacity is the slope of. Heat Capacity Entropy Equation.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID2494131 Heat Capacity Entropy Equation Consider adding a fixed amount of heat to a. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. the heat capacity is the slope of internal energy u with temperature (at constant volume) cv = (∂u∂t)v and the internal. heat capacities in. Heat Capacity Entropy Equation.
From www.researchgate.net
Heat capacity and entropy of a twostate system as a function of the Heat Capacity Entropy Equation measurements of the heat capacity of a substance and the enthalpies of fusion or vaporization can be used to calculate the changes in entropy that. the second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous. the heat flow is calculated from the first law of thermodynamics,. Heat Capacity Entropy Equation.
From www.grc.nasa.gov
Entropy of a Gas Heat Capacity Entropy Equation learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. heat capacities in enthalpy and entropy calculations. the heat flow is calculated from the first law of thermodynamics, q = δeint − w where δeint = 3 2nrδt for monatomic gasses. measurements of the heat capacity of a substance. Heat Capacity Entropy Equation.