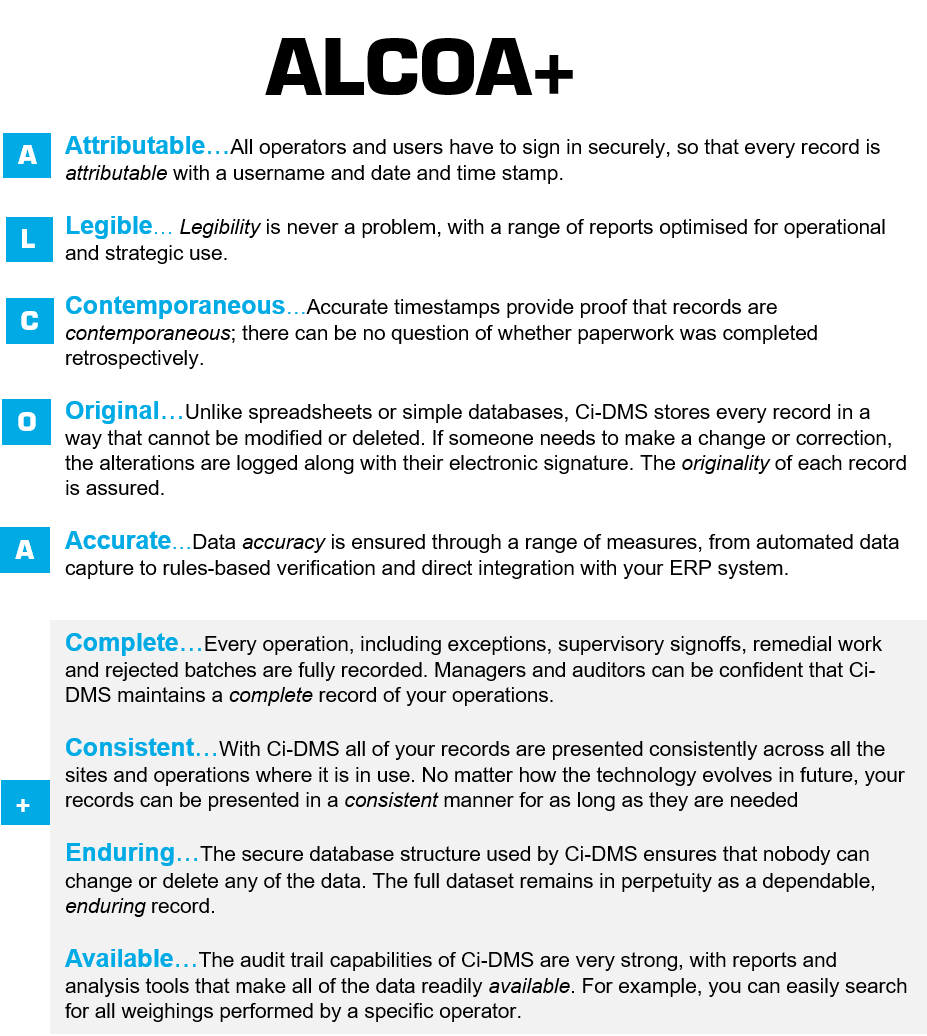
What Are The Alcoa Principles . Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. The acronym, which has expanded over the. By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Developed in the 1990s by. Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. Discover why it is so important. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. Record the person or system that performed an activity. What are the 9 principles of alcoa plus? Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Alcoa is an acronym based on the following five principles:
from www.vrogue.co
To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Developed in the 1990s by. What are the 9 principles of alcoa plus? By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. Record the person or system that performed an activity. Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. Alcoa is an acronym based on the following five principles: Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces.
Alcoa Plus And Alcoa Its Importance In Data Integrity vrogue.co
What Are The Alcoa Principles Record the person or system that performed an activity. Discover why it is so important. Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. Alcoa is an acronym based on the following five principles: What are the 9 principles of alcoa plus? By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. Record the person or system that performed an activity. Developed in the 1990s by. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. The acronym, which has expanded over the.
From www.labmanager.com
Lab Data Integrity and Security Best Practices Lab Manager What Are The Alcoa Principles By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. What are the 9 principles of alcoa plus? To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. The acronym, which has expanded over the. Discover why it is so important. Alcoa defines. What Are The Alcoa Principles.
From www.vrogue.co
Alcoa Plus And Alcoa Its Importance In Data Integrity vrogue.co What Are The Alcoa Principles Alcoa is an acronym based on the following five principles: By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete,. What Are The Alcoa Principles.
From scw.ai
ALCOA+ Data Integrity Guide for Pharmaceutical Manufacturers What Are The Alcoa Principles Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Record. What Are The Alcoa Principles.
From learnaboutpharma.com
ALCOA Plus Principles and its importance to Data Integrity What Are The Alcoa Principles Developed in the 1990s by. Discover why it is so important. Alcoa is an acronym based on the following five principles: The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. What are the 9 principles of alcoa plus? Alcoa is a set of guiding principles outlined by the fda that helps govern data. What Are The Alcoa Principles.
From totallab.com
ALCOA Principles in Life Sciences and Pharmaceutical Production TotalLab What Are The Alcoa Principles Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. Developed in the 1990s by. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete,. What Are The Alcoa Principles.
From www.pharmaguideline.co.uk
ALCOA Plus and ALCOA its importance in data integrity What Are The Alcoa Principles Discover why it is so important. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. What are the 9 principles of alcoa plus? Alcoa is an acronym based on the following five principles: To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa is a. What Are The Alcoa Principles.
From datamyte.com
ALCOA Principles A Comprehensive Guide DataMyte What Are The Alcoa Principles Developed in the 1990s by. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. The acronym, which has expanded over. What Are The Alcoa Principles.
From www.spectroscopyonline.com
Is Traceability the Glue for ALCOA, ALCOA+, or ALCOA++? What Are The Alcoa Principles To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. What are the 9 principles of alcoa plus? Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Discover why it is so. What Are The Alcoa Principles.
From learnaboutpharma.com
ALCOA Plus Principles and its importance to Data Integrity What Are The Alcoa Principles The acronym, which has expanded over the. Alcoa is an acronym based on the following five principles: Record the person or system that performed an activity. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in. What Are The Alcoa Principles.
From slcontrols.com
What is ALCOA+ and Why Is It Important to Validation and Data Integrity SL Controls What Are The Alcoa Principles Alcoa is an acronym based on the following five principles: Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Record the person or system that performed an activity. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. Alcoa defines how data should be attributable, legible,. What Are The Alcoa Principles.
From www.slideteam.net
Data Integrity Maintenance With Alcoa Principle PPT Sample What Are The Alcoa Principles Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Record the person or system that performed an activity. Alcoa is a set of guiding principles outlined by the fda that. What Are The Alcoa Principles.
From conductscience.com
ALCOAC Conduct Science What Are The Alcoa Principles Alcoa is an acronym based on the following five principles: Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. Discover why it is so important. Record the person or system that performed an activity. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. Alcoa is a set of guiding. What Are The Alcoa Principles.
From learnaboutpharma.com
ALCOA Plus Principles and its importance to Data Integrity What Are The Alcoa Principles Alcoa is an acronym based on the following five principles: Discover why it is so important. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. The acronym, which has expanded over the. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa defines how data. What Are The Alcoa Principles.
From learnaboutpharma.com
ALCOA Plus Principles and its importance to Data Integrity What Are The Alcoa Principles By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Discover why it is so important. Record the person or system that performed an activity. Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. Developed in the 1990s by. What are the 9 principles of alcoa. What Are The Alcoa Principles.
From learnaboutpharma.com
ALCOA Plus Principles and its importance to Data Integrity What Are The Alcoa Principles Record the person or system that performed an activity. What are the 9 principles of alcoa plus? Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. Developed in the 1990s by. Alcoa is a set of. What Are The Alcoa Principles.
From www.vrogue.co
What Is Data Integrity And Alcoa Plus vrogue.co What Are The Alcoa Principles The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Developed in the 1990s by. By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Alcoa is. What Are The Alcoa Principles.
From informacionpublica.svet.gob.gt
ALCOA Plus Principles For Data Integrity What Are The Alcoa Principles What are the 9 principles of alcoa plus? Record the person or system that performed an activity. The acronym, which has expanded over the. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. Alcoa is a set of principles established. What Are The Alcoa Principles.
From learnaboutpharma.com
ALCOA Plus Principles and its importance to Data Integrity What Are The Alcoa Principles Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. Record the person or system that performed an activity. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa. What Are The Alcoa Principles.
From www.cognidox.com
Data integrity in life sciences the vital role of ALCOA principles What Are The Alcoa Principles By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Record the person or system that performed an activity. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. Alcoa is a set of principles established by the fda to. What Are The Alcoa Principles.
From florencehc.com
ALCOAC in Clinical Trial Electronic Document Management Florence What Are The Alcoa Principles The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Developed in the 1990s by. Record the. What Are The Alcoa Principles.
From qmsadvisor.com
Understanding ALCOA Principles in Pharmaceutical Data Integrity Key to FDA Compliance 2024 What Are The Alcoa Principles Record the person or system that performed an activity. Discover why it is so important. Alcoa is an acronym based on the following five principles: By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. The acronym, which has expanded over the. Alcoa++ is a set of principles and guidelines. What Are The Alcoa Principles.
From www.pharmaqualification.com
Alcoa Plus Principles 4 More Terms to Consider What Are The Alcoa Principles Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. What. What Are The Alcoa Principles.
From www.slideserve.com
PPT Source Documentation PowerPoint Presentation, free download ID6642847 What Are The Alcoa Principles To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. Record the person or system that performed an activity. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. Developed. What Are The Alcoa Principles.
From expeed.com
Data Integrity Types, Threats, and Best Practices What Are The Alcoa Principles By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Developed in the 1990s by. Discover why it is so important. Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and.. What Are The Alcoa Principles.
From www.youtube.com
ALCOA and ALCOA+ in Pharmaceuticals Principles of ALCOA Data Integrity Principles YouTube What Are The Alcoa Principles Record the person or system that performed an activity. By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Developed in the 1990s by. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa++ is a set of principles and guidelines used. What Are The Alcoa Principles.
From www.learnaboutpharma.com
What is ALCOA Plus Principles And Its Importance To Data Integrity What Are The Alcoa Principles Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. Alcoa is an acronym based on the following five principles: Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. What are the 9 principles of alcoa plus? Alcoa++ is a set of principles and. What Are The Alcoa Principles.
From pharmaguddu.com
ALCOA to ALCOA Plus and Data integrity » Pharmaguddu What Are The Alcoa Principles Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Record the person or system that performed an activity. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa is an acronym based on the following five principles: Discover why it is so important. The. What Are The Alcoa Principles.
From pharmainterview.com
ALCOA Plus 9 Principles for Data Integrity What Are The Alcoa Principles Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity in the manufacturing industry. Developed in the 1990s by. By. What Are The Alcoa Principles.
From learnaboutpharma.com
ALCOA Plus Principles and its importance to Data Integrity What Are The Alcoa Principles Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. The acronym, which has expanded over the. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. What are the 9 principles of alcoa plus? Alcoa is a set of principles established by the fda to ensure data integrity and good. What Are The Alcoa Principles.
From www.pharmaudyog.com
ALCOA & ALCOA Plus Importance of Data integrity in Pharmaceutical industry Pharma Udyog What Are The Alcoa Principles Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Developed in the 1990s by. What are the 9 principles of alcoa plus? By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Record the person or system that performed an activity. Alcoa. What Are The Alcoa Principles.
From www.vrogue.co
Data Integrity And Principles Of Alcoa And Alcoa Api vrogue.co What Are The Alcoa Principles Discover why it is so important. The full form of alcoa + is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and. Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. Alcoa is a set of guiding principles outlined by the fda that helps govern data integrity. What Are The Alcoa Principles.
From www.youtube.com
ALCOA Principles YouTube What Are The Alcoa Principles Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Alcoa is a set of principles established by the fda to ensure data integrity and good documentation practices in the pharmaceutical industry. By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Alcoa. What Are The Alcoa Principles.
From www.clinical.ly
ALCOA+ and Data Integrity in Clinical Trials Clinical.ly What Are The Alcoa Principles Alcoa defines how data should be attributable, legible, contemporaneously recorded, original, and accurate. The acronym, which has expanded over the. Alcoa is an acronym based on the following five principles: To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. Alcoa is a set of guiding principles outlined by the fda that helps. What Are The Alcoa Principles.
From www.yokogawa.com
Simplifying pharma through digitization Yokogawa America What Are The Alcoa Principles Record the person or system that performed an activity. Alcoa is an acronym based on the following five principles: The acronym, which has expanded over the. By doing so, we can gain a clearer understanding of its significance in maintaining data integrity within the manufacturing industry. Alcoa++ is a set of principles and guidelines used in the life sciences and. What Are The Alcoa Principles.
From ciqa.net
Understanding FDA ALCOA Guidance for Data Integrity. What Are The Alcoa Principles Alcoa++ is a set of principles and guidelines used in the life sciences and other regulated spaces. Record the person or system that performed an activity. What are the 9 principles of alcoa plus? To grasp the concept more effectively, let’s dive into alcoa+ and explore each component of this acronym. The acronym, which has expanded over the. Developed in. What Are The Alcoa Principles.