Fda Water For Pharmaceutical Use . pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. This itg will cover the different types of water. this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. the type of water for pharmaceutical use is determined by usp testing.
from aqua-chem.com
(a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. This itg will cover the different types of water. the type of water for pharmaceutical use is determined by usp testing. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of.
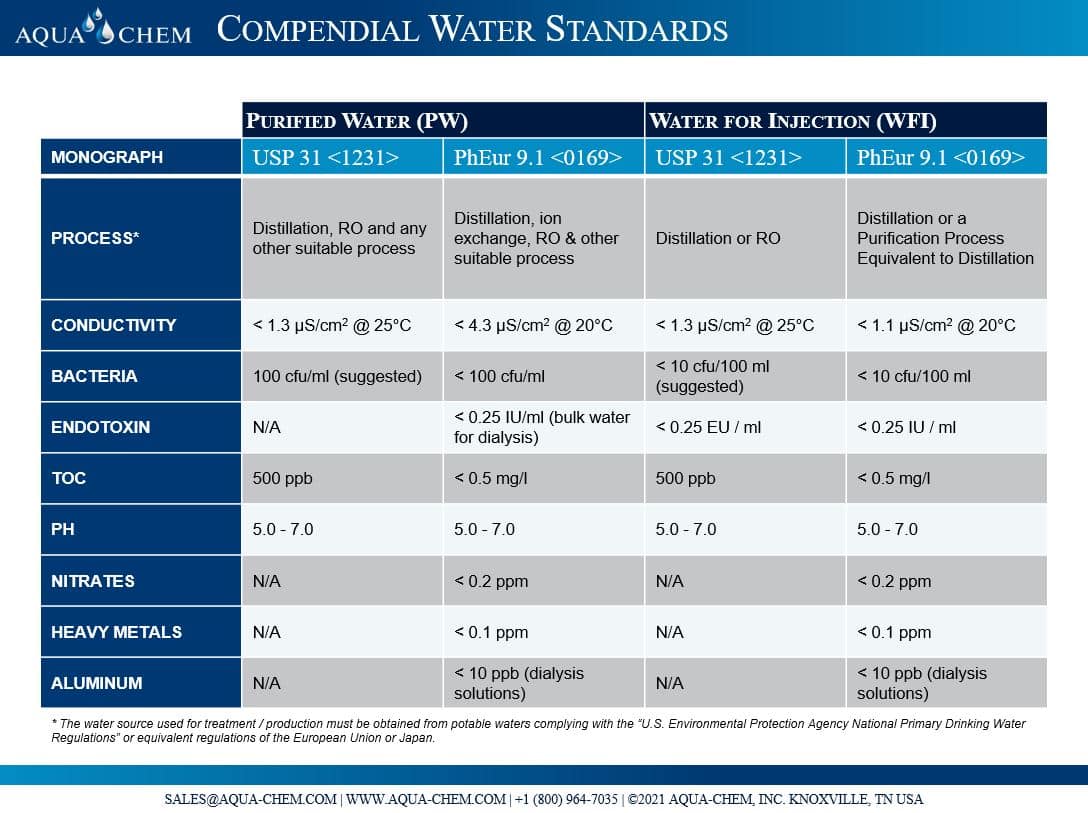
What are the differences between Purified Water (PW) and Water for Injection (WFI)? AQUACHEM
Fda Water For Pharmaceutical Use the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. This itg will cover the different types of water. the type of water for pharmaceutical use is determined by usp testing. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical.
From frestedt.com
Quality of Water for Pharmaceutical Use Frestedt Incorporated Fda Water For Pharmaceutical Use (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the.. Fda Water For Pharmaceutical Use.
From studylib.net
Water for Pharmaceutical Use Lecture 2 Water purification Fda Water For Pharmaceutical Use the type of water for pharmaceutical use is determined by usp testing. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. This itg will cover the different types of water. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. the type of water for pharmaceutical use is determined by usp testing. pharmaceutical water must be suitable for its intended. Fda Water For Pharmaceutical Use.
From www.pbahealth.com
Will New USP Water Purity Requirements Affect Your Independent Pharmacy? PBA Health Fda Water For Pharmaceutical Use This itg will cover the different types of water. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. the quality of water at the true point of use, as delivered. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use This itg will cover the different types of water. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. the quality of water at the true point of use, as delivered by. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use the type of water for pharmaceutical use is determined by usp testing. This itg will cover the different types of water. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. this. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. the type of water for pharmaceutical use is determined by usp testing. the quality of water at the true point of. Fda Water For Pharmaceutical Use.
From pharmaegg.com
Water Treatment in Pharmaceuticals Plant SOPs Pharma Egg Fda Water For Pharmaceutical Use the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. This itg will cover the different types of water. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. (a) potable water shall be supplied under continuous. Fda Water For Pharmaceutical Use.
From newdruginfo.com
1231WATER FOR PHARMACEUTICAL PURPOSES, chemical structure, molecular formula, Reference Standards Fda Water For Pharmaceutical Use This itg will cover the different types of water. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. pharmaceutical water must be suitable for its intended use and routinely tested to. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 3 Inspection of water purification systems PowerPoint Fda Water For Pharmaceutical Use the type of water for pharmaceutical use is determined by usp testing. this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. pharmaceutical water must be suitable. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 4 Commissioning, Qualification and validation Fda Water For Pharmaceutical Use This itg will cover the different types of water. (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. to ensure adherence to certain minimal chemical and microbiological quality standards, water used. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 4 Commissioning, Qualification and validation Fda Water For Pharmaceutical Use this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. the type of water for pharmaceutical use is determined by usp testing. (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. This itg will cover the. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use the type of water for pharmaceutical use is determined by usp testing. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. (a) potable water shall. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Pharmaceutical Water Systems PowerPoint Presentation, free download ID464322 Fda Water For Pharmaceutical Use to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. . Fda Water For Pharmaceutical Use.
From tsaprocessequipments.com
Understanding Purified Water for Pharmaceutical Applications Fda Water For Pharmaceutical Use (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. This itg will cover the different types of water. the quality of water at the true point. Fda Water For Pharmaceutical Use.
From newdruginfo.com
1231WATER FOR PHARMACEUTICAL PURPOSES, chemical structure, molecular formula, Reference Standards Fda Water For Pharmaceutical Use to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. guidance about which quality. Fda Water For Pharmaceutical Use.
From swaninstruments.ch
Online Pharmaceutical Water Monitoring Swan Analytical Instruments Fda Water For Pharmaceutical Use pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. this guide discusses,. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Pharmaceutical Water Systems PowerPoint Presentation, free download ID733349 Fda Water For Pharmaceutical Use the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. the type of water for pharmaceutical use is determined by usp testing. This itg will cover the different. Fda Water For Pharmaceutical Use.
From www.pharmamicroresources.com
Pharmaceutical Microbiology Fda Water For Pharmaceutical Use the type of water for pharmaceutical use is determined by usp testing. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. (a) potable water shall be supplied under continuous positive. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Pharmaceutical Water Systems PowerPoint Presentation, free download ID464322 Fda Water For Pharmaceutical Use (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the.. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. the quality of water at the true point of use, as delivered by manufacturing (or by a. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use PowerPoint Presentation, free download ID1113782 Fda Water For Pharmaceutical Use to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. This itg will cover the different types of water. (a) potable water shall be supplied under continuous positive. Fda Water For Pharmaceutical Use.
From pharmait.dk
Guidance on Quality of Water for Pharmaceutical Use Pharma IT Insights Fda Water For Pharmaceutical Use pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. the type of water for pharmaceutical use is determined by usp testing. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. (a) potable water shall be supplied under continuous positive pressure. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. to ensure adherence. Fda Water For Pharmaceutical Use.
From kremesti.com
Ultra Pure Water Treatment for Pharmaceutical Industry Fda Water For Pharmaceutical Use to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. . Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. This itg will cover the different types of water. this guide discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the. the quality of water at the true point of. Fda Water For Pharmaceutical Use.
From aqua-chem.com
What are the differences between Purified Water (PW) and Water for Injection (WFI)? AQUACHEM Fda Water For Pharmaceutical Use to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. This itg will cover the different types of water. the type of water for pharmaceutical use is determined by usp testing. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process. Fda Water For Pharmaceutical Use.
From www.scribd.com
Water For Pharmaceutical Use Purified Water Water Purification Fda Water For Pharmaceutical Use the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. pharmaceutical water must be suitable for its intended use and routinely tested to ensure ongoing conformance with.. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 4 Commissioning, Qualification and validation Fda Water For Pharmaceutical Use (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. guidance about which quality of water to use for specic applications, such as the manufacture of active. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use This itg will cover the different types of water. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. the type of water for pharmaceutical use is determined by usp testing. to ensure adherence to certain minimal chemical and microbiological quality standards, water used in. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 1 Introduction and treatment PowerPoint Presentation Fda Water For Pharmaceutical Use the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. This itg will cover the different types of water. to ensure adherence to certain minimal chemical and. Fda Water For Pharmaceutical Use.
From www.johner-institut.de
FDA Warning Letters und Formular 483 Fda Water For Pharmaceutical Use to ensure adherence to certain minimal chemical and microbiological quality standards, water used in the production of. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the.. Fda Water For Pharmaceutical Use.
From awinhospital.en.made-in-china.com
High Quality FDA Standard cGMP MultiEffect Distilled Water Making Machine for Pharmaceutical Fda Water For Pharmaceutical Use the type of water for pharmaceutical use is determined by usp testing. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. (a) potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that. pharmaceutical water must be suitable for its intended. Fda Water For Pharmaceutical Use.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 3 Inspection of water purification systems PowerPoint Fda Water For Pharmaceutical Use This itg will cover the different types of water. the quality of water at the true point of use, as delivered by manufacturing (or by a sampling process identical to the. guidance about which quality of water to use for specic applications, such as the manufacture of active pharmaceutical. (a) potable water shall be supplied under continuous. Fda Water For Pharmaceutical Use.