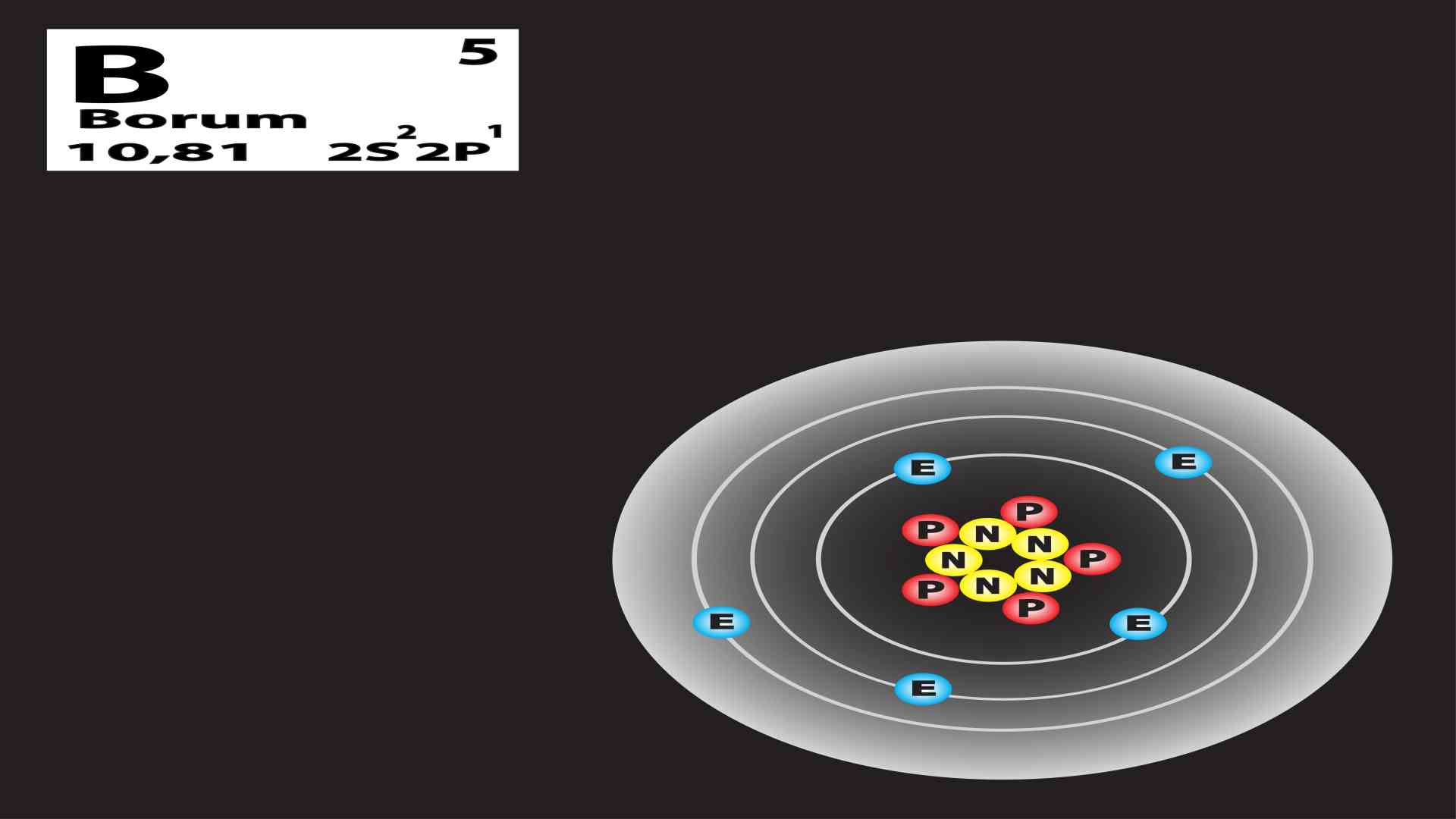
Why Boron 10 And Boron 11 Are Isotopes . The atomic nucleus consists of 5 neutrons and the element. The atomic mass of boron is 10.81 u. After neutron capture, the 10. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. Allotropes are forms of an element with different physical and chemical properties. It has a relative isotopic mass of 10 and consists of.
from borates.today
It has a relative isotopic mass of 10 and consists of. The atomic nucleus consists of 5 neutrons and the element. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. Allotropes are forms of an element with different physical and chemical properties. After neutron capture, the 10. The atomic mass of boron is 10.81 u. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors.
Boron Chemistry Borates Today
Why Boron 10 And Boron 11 Are Isotopes Allotropes are forms of an element with different physical and chemical properties. It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element with different physical and chemical properties. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. The atomic nucleus consists of 5 neutrons and the element. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. After neutron capture, the 10. The atomic mass of boron is 10.81 u. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter.
From www.numerade.com
SOLVED The two naturally occurring isotopes of boron, boron10 and boron11, have masses of10 Why Boron 10 And Boron 11 Are Isotopes Slight variations in this proportion produce a range of ±0.003 in the atomic weight. It has a relative isotopic mass of 10 and consists of. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. Allotropes are forms of an element with different physical and chemical properties. The atomic nucleus consists of. Why Boron 10 And Boron 11 Are Isotopes.
From borates.today
Boron Chemistry Borates Today Why Boron 10 And Boron 11 Are Isotopes It has a relative isotopic mass of 10 and consists of. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. After neutron capture, the 10. Allotropes are forms of an element with different physical and chemical properties. The atomic mass of boron is 10.81 u. The atomic nucleus consists of 5. Why Boron 10 And Boron 11 Are Isotopes.
From byjus.com
boron exist as two isotopes ^10B and ^11B if atomic mass of boron in periodic table is given to Why Boron 10 And Boron 11 Are Isotopes One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. It has a relative isotopic mass of 10 and consists of. The atomic mass of boron is 10.81 u. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication. Why Boron 10 And Boron 11 Are Isotopes.
From www.youtube.com
Boron has two stable isotopes, 10B (19) and 11B (81). Calculate average at. wt. of boron Why Boron 10 And Boron 11 Are Isotopes To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. The atomic mass of boron is 10.81 u. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. One form of boron consists of clear red crystals with a density of. Why Boron 10 And Boron 11 Are Isotopes.
From slideplayer.com
Metals and Non Metals. ppt download Why Boron 10 And Boron 11 Are Isotopes One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. The atomic mass of boron is 10.81 u. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. Allotropes are forms of an element with different physical. Why Boron 10 And Boron 11 Are Isotopes.
From www.nuclear-power.com
Boron Atomic Number Atomic Mass Density of Boron Why Boron 10 And Boron 11 Are Isotopes The atomic nucleus consists of 5 neutrons and the element. It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element with different physical and chemical properties. After neutron capture, the 10. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. To enhance the conversion. Why Boron 10 And Boron 11 Are Isotopes.
From slideplayer.com
Physical Science Chemistry Unit 2 ppt download Why Boron 10 And Boron 11 Are Isotopes One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. Allotropes are forms of an element with different physical and chemical properties. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. The atomic mass of boron is 10.81 u. To enhance the conversion efficiency of lithium or. Why Boron 10 And Boron 11 Are Isotopes.
From www.alamy.com
boron isotopes atomic structure backdrop physics theory illustration schematic Stock Photo Alamy Why Boron 10 And Boron 11 Are Isotopes After neutron capture, the 10. Allotropes are forms of an element with different physical and chemical properties. The atomic mass of boron is 10.81 u. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. The atomic nucleus consists of 5 neutrons and the element. It has a relative isotopic mass of. Why Boron 10 And Boron 11 Are Isotopes.
From www.numerade.com
SOLVED Question 88 (2 points) There are two stable isotopes of boron boron10 and boron11 Why Boron 10 And Boron 11 Are Isotopes The atomic mass of boron is 10.81 u. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. After neutron capture, the 10. Allotropes are forms of an element with different physical and chemical properties. It has a relative isotopic mass of 10 and consists of. To enhance the conversion efficiency of. Why Boron 10 And Boron 11 Are Isotopes.
From www.alamy.com
boron isotopes atomic structure backdrop physics theory illustration schematic Stock Photo Alamy Why Boron 10 And Boron 11 Are Isotopes It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element with different physical and chemical properties. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. To enhance the conversion efficiency. Why Boron 10 And Boron 11 Are Isotopes.
From ar.inspiredpencil.com
Boron Isotopes Why Boron 10 And Boron 11 Are Isotopes To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element. Why Boron 10 And Boron 11 Are Isotopes.
From www.alamy.com
boron isotopes atomic structure backdrop physics theory illustration schematic Stock Photo Alamy Why Boron 10 And Boron 11 Are Isotopes The atomic mass of boron is 10.81 u. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. The atomic nucleus consists of 5 neutrons and the element. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. To enhance the conversion efficiency of lithium or boron, samples. Why Boron 10 And Boron 11 Are Isotopes.
From www.slideserve.com
PPT Atoms, ions & isotopes Click on mouse or press ‘Enter’ to begin. PowerPoint Presentation Why Boron 10 And Boron 11 Are Isotopes The atomic nucleus consists of 5 neutrons and the element. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element with different physical and chemical properties. The atomic mass of boron is 10.81 u. After. Why Boron 10 And Boron 11 Are Isotopes.
From www.numerade.com
SOLVED Isotopes of Boron Boron contains 10B (10.0129g/mol) and 11^B. 10^B is 19.91 abundant Why Boron 10 And Boron 11 Are Isotopes After neutron capture, the 10. The atomic mass of boron is 10.81 u. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. The atomic nucleus consists of 5 neutrons and the element. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often. Why Boron 10 And Boron 11 Are Isotopes.
From www.transtutors.com
(Solved) boron has two naturally occurring isotopes b10 with an isotopic... (1 Answer Why Boron 10 And Boron 11 Are Isotopes After neutron capture, the 10. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. It has a relative isotopic mass of 10 and consists of. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. Allotropes. Why Boron 10 And Boron 11 Are Isotopes.
From www.slideserve.com
PPT NUCLEAR CHEMISTRY PowerPoint Presentation, free download ID3795675 Why Boron 10 And Boron 11 Are Isotopes Allotropes are forms of an element with different physical and chemical properties. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. It has a relative isotopic mass of 10 and consists of. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. The atomic mass of boron. Why Boron 10 And Boron 11 Are Isotopes.
From nscl.msu.edu
Convenient location of a nearthreshold protonemitting resonance in boron11 National Why Boron 10 And Boron 11 Are Isotopes After neutron capture, the 10. The atomic nucleus consists of 5 neutrons and the element. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. The atomic mass of boron is 10.81 u. It has a relative isotopic. Why Boron 10 And Boron 11 Are Isotopes.
From www.numerade.com
SOLVED Naturally occurring boron consists of two isotopes with the isotopic masses 10.013 and Why Boron 10 And Boron 11 Are Isotopes It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element with different physical and chemical properties. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. Slight variations in this proportion produce a range of ±0.003 in the. Why Boron 10 And Boron 11 Are Isotopes.
From www.youtube.com
Boron has two isotopes, B10 and B11. The average atomic mass of boron is found to be \( 10.80 Why Boron 10 And Boron 11 Are Isotopes One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. After neutron capture, the 10. It has a relative isotopic mass of 10 and consists of. The atomic nucleus consists of 5 neutrons and the element. Allotropes are forms of an element with different physical and chemical properties. The atomic mass of. Why Boron 10 And Boron 11 Are Isotopes.
From fyovszriu.blob.core.windows.net
How Do Boron 10 And Boron 11 Differ at Joseph Pokorny blog Why Boron 10 And Boron 11 Are Isotopes To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. The atomic mass of boron is 10.81 u. After neutron capture, the 10. It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element with different physical and chemical. Why Boron 10 And Boron 11 Are Isotopes.
From www.numerade.com
SOLVED Boron obtained from borax deposits in Death Valley consists of two isotopes, They are Why Boron 10 And Boron 11 Are Isotopes One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. Allotropes are forms of an element with different physical and chemical properties. After neutron capture, the 10. The atomic nucleus consists of 5 neutrons and the element. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. To. Why Boron 10 And Boron 11 Are Isotopes.
From www.goodscience.com.au
Atomic Number, Mass Number and Isotopes Good Science Why Boron 10 And Boron 11 Are Isotopes After neutron capture, the 10. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. The atomic mass of boron is 10.81 u. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. Allotropes are forms of an element with different. Why Boron 10 And Boron 11 Are Isotopes.
From www.slideserve.com
PPT NUCLEAR CHEMISTRY PowerPoint Presentation, free download ID3795675 Why Boron 10 And Boron 11 Are Isotopes One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. After neutron capture, the 10. The atomic nucleus consists of 5 neutrons and the element. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. It has a relative isotopic mass of 10 and consists of. To enhance. Why Boron 10 And Boron 11 Are Isotopes.
From www.numerade.com
SOLVEDThe stable isotopes of boron are boron10 and boron11. Four radioactive isotopes with Why Boron 10 And Boron 11 Are Isotopes The atomic mass of boron is 10.81 u. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. After neutron capture, the 10. The atomic nucleus consists of 5 neutrons and. Why Boron 10 And Boron 11 Are Isotopes.
From www.numerade.com
The element boron has two stable isotopes boron10 (10.0129 u) and boron11 (11.0093 u). The Why Boron 10 And Boron 11 Are Isotopes Slight variations in this proportion produce a range of ±0.003 in the atomic weight. The atomic nucleus consists of 5 neutrons and the element. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element with. Why Boron 10 And Boron 11 Are Isotopes.
From www.alamy.com
boron isotopes atomic structure backdrop physics theory illustration schematic Stock Photo Alamy Why Boron 10 And Boron 11 Are Isotopes It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element with different physical and chemical properties. The atomic nucleus consists of 5 neutrons and the element. After neutron capture, the 10. The atomic mass of boron is 10.81 u. One form of boron consists of clear red crystals with a density of 2.46. Why Boron 10 And Boron 11 Are Isotopes.
From www.numerade.com
SOLVED 'In the previous example; You added 4 Boron11 isotopes to 1 Boron10 to make the Why Boron 10 And Boron 11 Are Isotopes The atomic nucleus consists of 5 neutrons and the element. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. Allotropes are forms of an element with different physical and chemical. Why Boron 10 And Boron 11 Are Isotopes.
From www.alamy.com
boron isotopes atomic structure illustration chemical element illustration schematic Stock Why Boron 10 And Boron 11 Are Isotopes After neutron capture, the 10. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. The atomic mass of boron is 10.81 u. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. Slight variations in this. Why Boron 10 And Boron 11 Are Isotopes.
From www.americanelements.com
Boron10 Isotope AMERICAN ELEMENTS Why Boron 10 And Boron 11 Are Isotopes Allotropes are forms of an element with different physical and chemical properties. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. The atomic mass of boron is 10.81 u. After. Why Boron 10 And Boron 11 Are Isotopes.
From slideplayer.com
Section 2 Masses of Atoms ppt download Why Boron 10 And Boron 11 Are Isotopes The atomic mass of boron is 10.81 u. Allotropes are forms of an element with different physical and chemical properties. It has a relative isotopic mass of 10 and consists of. After neutron capture, the 10. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. To enhance the conversion efficiency of lithium or boron, samples. Why Boron 10 And Boron 11 Are Isotopes.
From www.youtube.com
Boron has two isotopes, boron10 and boron11. Based on the average atomic mass, which isotope Why Boron 10 And Boron 11 Are Isotopes The atomic mass of boron is 10.81 u. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. After neutron capture, the 10. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. The atomic nucleus consists of 5 neutrons and. Why Boron 10 And Boron 11 Are Isotopes.
From pages.swcp.com
ON QUARKS, NUCLEI and BORON10 NEUTRON CAPTURE Why Boron 10 And Boron 11 Are Isotopes After neutron capture, the 10. Allotropes are forms of an element with different physical and chemical properties. One form of boron consists of clear red crystals with a density of 2.46 grams per cubic centimeter. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. It has a relative isotopic mass of 10 and consists of.. Why Boron 10 And Boron 11 Are Isotopes.
From www.techexplorist.com
Scientists determined the geometry of two isotopes of boron Why Boron 10 And Boron 11 Are Isotopes Slight variations in this proportion produce a range of ±0.003 in the atomic weight. The atomic mass of boron is 10.81 u. It has a relative isotopic mass of 10 and consists of. Allotropes are forms of an element with different physical and chemical properties. One form of boron consists of clear red crystals with a density of 2.46 grams. Why Boron 10 And Boron 11 Are Isotopes.
From www.toppr.com
Boron found in nature having atomic weight of 10.811 and is made up of the isotopes B^10 (mass Why Boron 10 And Boron 11 Are Isotopes The atomic mass of boron is 10.81 u. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. The atomic nucleus consists of 5 neutrons and the element. After neutron capture, the 10. Allotropes are forms of an element with different physical and chemical properties.. Why Boron 10 And Boron 11 Are Isotopes.
From www.slideserve.com
PPT Isotopes PowerPoint Presentation, free download ID1297106 Why Boron 10 And Boron 11 Are Isotopes The atomic mass of boron is 10.81 u. Slight variations in this proportion produce a range of ±0.003 in the atomic weight. The atomic nucleus consists of 5 neutrons and the element. It has a relative isotopic mass of 10 and consists of. After neutron capture, the 10. One form of boron consists of clear red crystals with a density. Why Boron 10 And Boron 11 Are Isotopes.