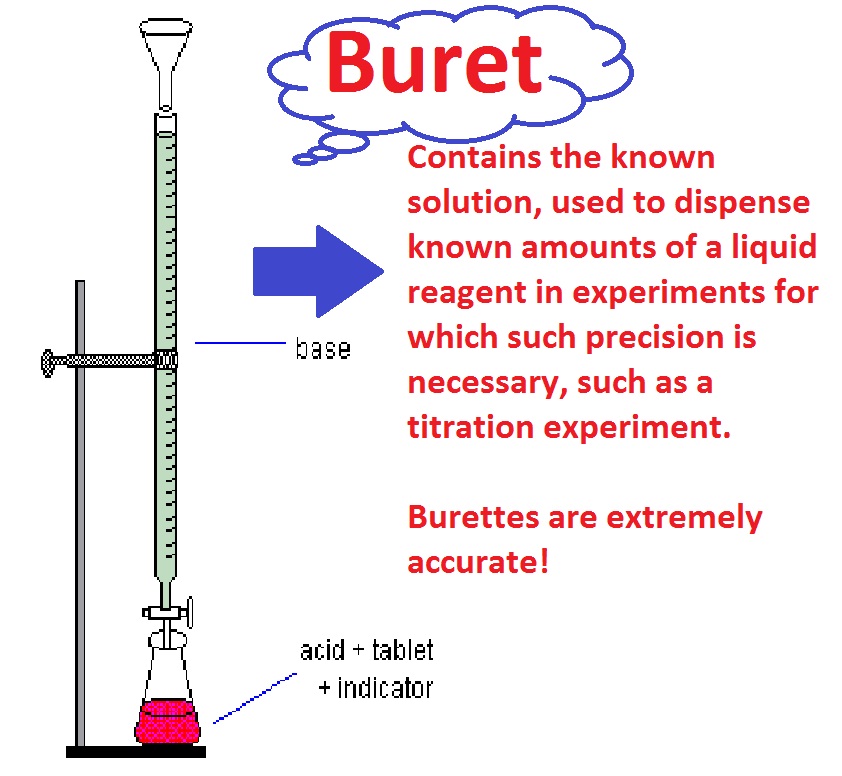
Titration Meaning Journal . Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or.
from chem4three.blogspot.co.uk
Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or.
CHEMISTRY 11 TITRATIONS
Titration Meaning Journal Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to. Titration Meaning Journal.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Meaning Journal Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Since not all reactions. Titration Meaning Journal.
From school.careers360.com
types of titration Overview, Structure, Properties & Uses Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. A titration. Titration Meaning Journal.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Meaning Journal Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Meaning Journal.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Meaning Journal A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration Meaning Journal.
From novapublishers.com
Titration Theory, Types, Techniques and Uses Nova Science Publishers Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Electrochemical titration, a. Titration Meaning Journal.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is the quantitative addition of. Titration Meaning Journal.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is an. Titration Meaning Journal.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is the slow addition of one solution. Titration Meaning Journal.
From www.scribd.com
Types of Titration Titration Redox Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Meaning Journal.
From www.slideserve.com
PPT Intro to Titrations PowerPoint Presentation, free download ID Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is an analytical method used. Titration Meaning Journal.
From present5.com
Titration and AcidBase Neutralization LEARNING OBJECTIVES 11.2.2.7 Titration Meaning Journal Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is an analytical method used in biomedical sciences and analytical. Titration Meaning Journal.
From testbook.com
Karl Fischer Titration Know its Definition, Example, Procedure Titration Meaning Journal Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is an. Titration Meaning Journal.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Titration Meaning Journal A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is the. Titration Meaning Journal.
From www.studypool.com
SOLUTION Titrations titration meaning Studypool Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is an analytical. Titration Meaning Journal.
From www.slideserve.com
PPT Chem. 31 2/3 Lecture PowerPoint Presentation, free download Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Meaning Journal.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Meaning Journal.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration. Titration Meaning Journal.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is an analytical method used. Titration Meaning Journal.
From www.studypool.com
SOLUTION Comprehensive explanation on titration from princeton Studypool Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is the quantitative addition. Titration Meaning Journal.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Titration is an analytical method used in biomedical sciences and analytical chemistry. Titration Meaning Journal.
From chem4three.blogspot.co.uk
CHEMISTRY 11 TITRATIONS Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Meaning Journal.
From www.studypool.com
SOLUTION Comprehensive explanation on titration from princeton Studypool Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is an analytical. Titration Meaning Journal.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is an analytical method used. Titration Meaning Journal.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed. Titration Meaning Journal.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Meaning Journal.
From www.rdworldonline.com
What are titration instruments? Research & Development World Titration Meaning Journal Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Meaning Journal.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Since not all reactions fulfil the desired conditions, in practice various titration strategies have. Titration Meaning Journal.
From www.studocu.com
Titration Curves Interpretation of titration curves strong Acid Titration Meaning Journal A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Electrochemical. Titration Meaning Journal.
From labthink.netlify.app
What is titration and how does it work Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Since not all reactions fulfil the. Titration Meaning Journal.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is the quantitative addition of a solution. Titration Meaning Journal.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Titration Meaning Journal Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration Meaning Journal.
From www.pinterest.com
Titration Easy Science Learn biology, Cool science facts, Ap chemistry Titration Meaning Journal Since not all reactions fulfil the desired conditions, in practice various titration strategies have been developed in order to be. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is an analytical. Titration Meaning Journal.
From byjus.com
What is the meaning of titration?? Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to. Titration Meaning Journal.
From slideplayer.com
U2 S3 L2 Titration page 603 AcidBase Titration pages Titration Step Titration Meaning Journal Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or. Electrochemical titration, a direct method for generating standard solutions, relies on faraday's law to establish a direct relationship. Titration is the quantitative addition of a solution of known concentration to a solution of unknown concentration until the reaction. Titration is an analytical. Titration Meaning Journal.