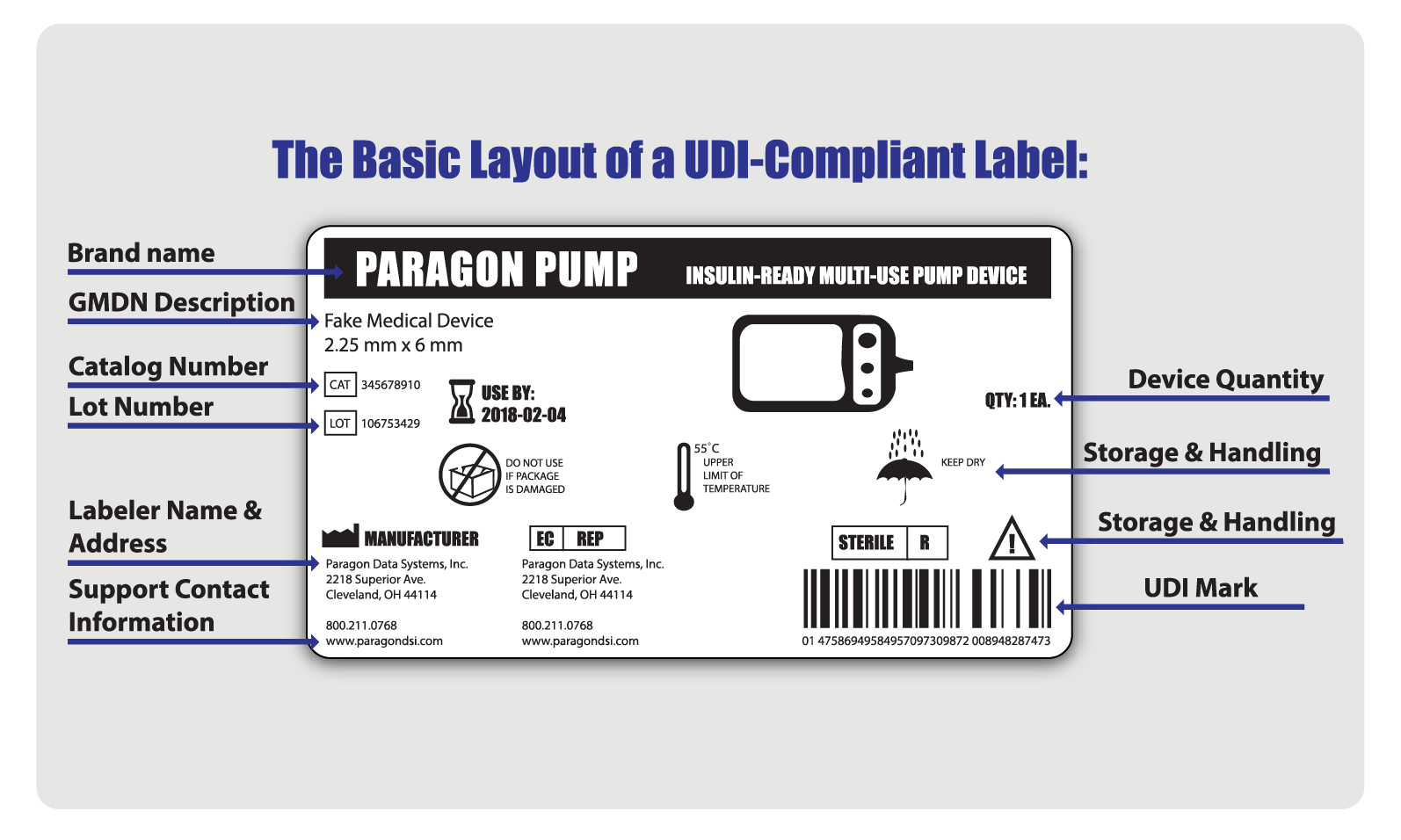
Labelling Requirements For Medical Devices In India . The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. What are the labeling requirements for medical devices in india? Asia actual has established a system for the local labeling of registered medical devices in india prior to customs clearance. The drugs and cosmetics act, 1940, and rules regulate all medical. Product labels shall comply with the labelling requirements as prescribed in. India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for.
from www.vrogue.co
Asia actual has established a system for the local labeling of registered medical devices in india prior to customs clearance. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Product labels shall comply with the labelling requirements as prescribed in. India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the. The drugs and cosmetics act, 1940, and rules regulate all medical. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. What are the labeling requirements for medical devices in india?
Medical Device Labeling Requirements What You Need To vrogue.co
Labelling Requirements For Medical Devices In India The drugs and cosmetics act, 1940, and rules regulate all medical. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. What are the labeling requirements for medical devices in india? Asia actual has established a system for the local labeling of registered medical devices in india prior to customs clearance. The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Product labels shall comply with the labelling requirements as prescribed in. The drugs and cosmetics act, 1940, and rules regulate all medical. India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the.
From medicaldevicelicense.com
Medical Device Blog I Discover Latest Expert Analysis of MDR Labelling Requirements For Medical Devices In India The drugs and cosmetics act, 1940, and rules regulate all medical. Product labels shall comply with the labelling requirements as prescribed in. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Ministry of health & family welfare, government of india has. Labelling Requirements For Medical Devices In India.
From www.slideserve.com
PPT Medical Device Labelling Regulatory Solutions India PowerPoint Labelling Requirements For Medical Devices In India India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the. The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. Asia actual has established a system for the local labeling of registered medical devices in. Labelling Requirements For Medical Devices In India.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Labelling Requirements For Medical Devices In India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. The drugs and cosmetics act, 1940, and rules regulate all medical. The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. India is required to be made by the manufacturer or importer or his agent in. Labelling Requirements For Medical Devices In India.
From www.afpharmaservice.com
Medical Device Labelling Requirements Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Product labels shall comply with the labelling requirements as prescribed in. India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in. Labelling Requirements For Medical Devices In India.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 The drugs and cosmetics act, 1940, and rules regulate all medical. The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. Asia actual has established a system. Labelling Requirements For Medical Devices In India.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Labelling Requirements For Medical Devices In India Product labels shall comply with the labelling requirements as prescribed in. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Asia actual has established a system for the local labeling of registered medical devices in india prior to customs clearance. What are the labeling requirements for medical devices in india?. Labelling Requirements For Medical Devices In India.
From mungfali.com
FDA Medical Device Label Symbols Labelling Requirements For Medical Devices In India The drugs and cosmetics act, 1940, and rules regulate all medical. Asia actual has established a system for the local labeling of registered medical devices in india prior to customs clearance. Product labels shall comply with the labelling requirements as prescribed in. The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. Ministry of health. Labelling Requirements For Medical Devices In India.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Labelling Requirements For Medical Devices In India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. What are the labeling requirements for medical devices in india? India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the. 89. Labelling Requirements For Medical Devices In India.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Requirements For Medical Devices In India What are the labeling requirements for medical devices in india? 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018. Labelling Requirements For Medical Devices In India.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Labelling Requirements For Medical Devices In India Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. Product labels shall comply with the labelling requirements as prescribed in. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. The drugs controller general (india) has notification. Labelling Requirements For Medical Devices In India.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. India is required to be made by the manufacturer or importer or his. Labelling Requirements For Medical Devices In India.
From www.slideserve.com
PPT Medical Device Labelling Regulatory Solutions India PowerPoint Labelling Requirements For Medical Devices In India Product labels shall comply with the labelling requirements as prescribed in. India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the. The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. Ministry of health &. Labelling Requirements For Medical Devices In India.
From mavink.com
Medical Device Labeling Symbols Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. India is required to be made by the manufacturer or importer or his agent in india, in. Labelling Requirements For Medical Devices In India.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Labelling Requirements For Medical Devices In India The drugs and cosmetics act, 1940, and rules regulate all medical. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. India is required to be made. Labelling Requirements For Medical Devices In India.
From old.sermitsiaq.ag
Medical Device Label Template Labelling Requirements For Medical Devices In India The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. Product labels shall comply with the labelling requirements as prescribed in. Asia actual has established a system for the local labeling of registered medical devices in india prior to customs clearance. India is required to be made by the manufacturer or importer or his agent. Labelling Requirements For Medical Devices In India.
From medicaldevices.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Medical Devices Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. The drugs and cosmetics act, 1940, and rules regulate all medical. What are. Labelling Requirements For Medical Devices In India.
From www.linkedin.com
Labelling Requirements for Medical Devices in India Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 The drugs and cosmetics act, 1940, and rules regulate all medical. India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in. Labelling Requirements For Medical Devices In India.
From www.tuvsud.com
India Medical Device Regulations TÜV SÜD South Africa Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. What are the labeling requirements for medical devices in. Labelling Requirements For Medical Devices In India.
From www.scribd.com
Guide To Mandatory Labelling Requirements For Cosmetics in India Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Asia actual has established a system for the local labeling of registered medical. Labelling Requirements For Medical Devices In India.
From www.slideserve.com
PPT Medical Device Labelling Regulatory Solutions India PowerPoint Labelling Requirements For Medical Devices In India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. What are the labeling requirements for medical devices in india? 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Product labels shall. Labelling Requirements For Medical Devices In India.
From www.pacificbridgemedical.com
Device Classification in India Infographic Labelling Requirements For Medical Devices In India The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 What are the labeling requirements for medical devices in india? Product labels shall comply with the labelling. Labelling Requirements For Medical Devices In India.
From cliniexperts.com
MoHFW Notification For Medical Devices Registration & Labelling, India Labelling Requirements For Medical Devices In India The drugs and cosmetics act, 1940, and rules regulate all medical. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 What are the labeling requirements for medical devices in india? Product labels shall comply with the labelling requirements as prescribed in.. Labelling Requirements For Medical Devices In India.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Labelling Requirements For Medical Devices In India Asia actual has established a system for the local labeling of registered medical devices in india prior to customs clearance. The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. The drugs and cosmetics act, 1940,. Labelling Requirements For Medical Devices In India.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Labelling Requirements For Medical Devices In India Asia actual has established a system for the local labeling of registered medical devices in india prior to customs clearance. India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the. Product labels shall comply with the labelling requirements as prescribed. Labelling Requirements For Medical Devices In India.
From e-startupindia.com
US FDA labelling requirements for medical devices EStartupIndia Labelling Requirements For Medical Devices In India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Product labels shall comply with the labelling requirements as prescribed in. Ministry of. Labelling Requirements For Medical Devices In India.
From operonstrategist.com
Medical Device Consulting Company in India Operon Strategist Labelling Requirements For Medical Devices In India What are the labeling requirements for medical devices in india? Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Asia actual has established a system for the. Labelling Requirements For Medical Devices In India.
From pharmadocx.com
MDR Labelling Requirements for Medical Devices in India Pharmadocx Labelling Requirements For Medical Devices In India India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. What are the labeling requirements for medical devices in india? The. Labelling Requirements For Medical Devices In India.
From www.slideserve.com
PPT Medical Device Labelling Regulatory Solutions India PowerPoint Labelling Requirements For Medical Devices In India The drugs and cosmetics act, 1940, and rules regulate all medical. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Product labels shall comply with the labelling requirements as prescribed in. Discover the essential medical device labeling requirements in india as. Labelling Requirements For Medical Devices In India.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Labelling Requirements For Medical Devices In India The drugs and cosmetics act, 1940, and rules regulate all medical. India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1. Labelling Requirements For Medical Devices In India.
From clin-r.com
Labels for Medical Devices Clin R Labelling Requirements For Medical Devices In India Asia actual has established a system for the local labeling of registered medical devices in india prior to customs clearance. What are the labeling requirements for medical devices in india? 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 India is. Labelling Requirements For Medical Devices In India.
From dokumen.tips
(PDF) UDI Labeling Requirements for Medical Devices Part II · UDI Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 The drugs and cosmetics act, 1940, and rules regulate all medical. The drugs controller general (india) has notification for medical devices pertaining to registration and labelling. Asia actual has established a system. Labelling Requirements For Medical Devices In India.
From clin-r.com
Labels for Medical Devices Clin R Labelling Requirements For Medical Devices In India 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. The drugs and cosmetics act, 1940, and rules regulate all medical. India is. Labelling Requirements For Medical Devices In India.
From apacmed.org
APACMed Position Paper with to Facilitate Adoption of Labelling Requirements For Medical Devices In India Product labels shall comply with the labelling requirements as prescribed in. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january,. Labelling Requirements For Medical Devices In India.
From www.tuvsud.com
Infographic The New Medical Device Regulation TÜV SÜD in India Labelling Requirements For Medical Devices In India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Product labels shall comply with the labelling requirements as prescribed in. 89 rows list of medical devices testing laboratory (mdtl) for carry out test or evaluation of medical device on behalf of manufacturer registered with cdsco under mdr 2017 India is. Labelling Requirements For Medical Devices In India.
From blog.nkgabc.com
Class A notified medical devices registration in India NKGABC Blog Labelling Requirements For Medical Devices In India What are the labeling requirements for medical devices in india? Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. India is required to be made by the manufacturer or importer or his agent in india, in form 40 and in form and manner as under rule 24a of the. Asia. Labelling Requirements For Medical Devices In India.