What Is Medical Device Complaints . 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and.
from www.pdffiller.com
No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry involves so much more than placating upset customers. 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,.
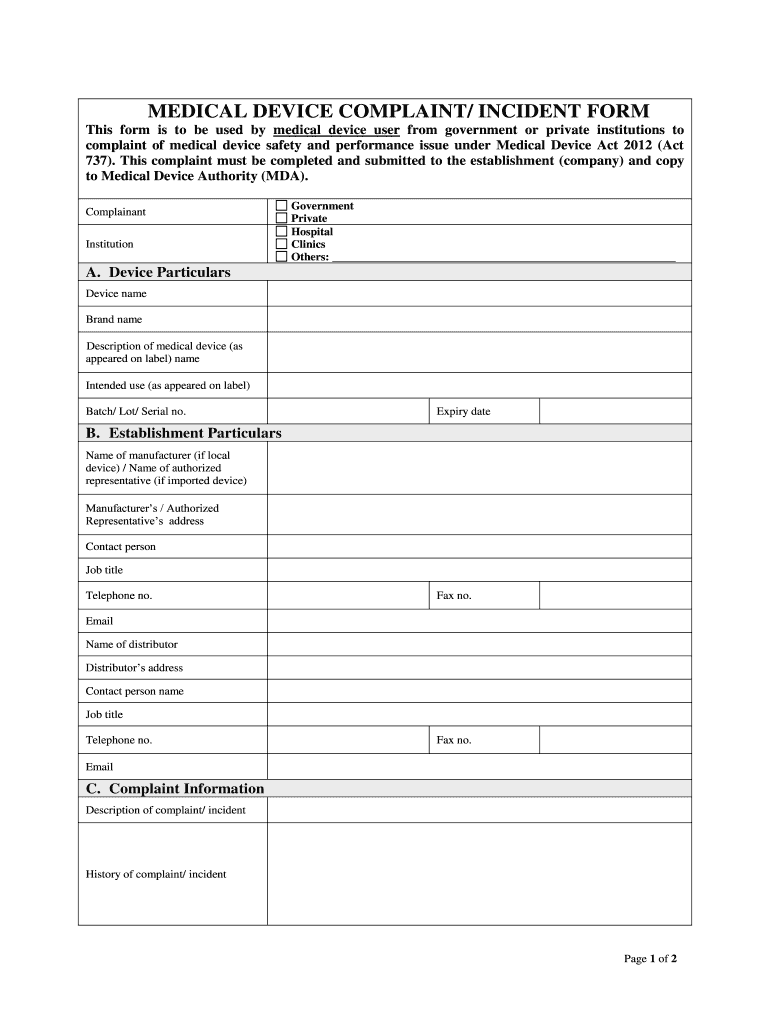
Fillable Online MEDICAL DEVICE COMPLAINT/ INCIDENT FORM Fax Email Print
What Is Medical Device Complaints In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry involves so much more than placating upset customers. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda.
From www.greenlight.guru
Medical Device Complaint Handling Procedure [+Flowchart] What Is Medical Device Complaints Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device reporting (mdr) regulation. What Is Medical Device Complaints.
From www.linkedin.com
5 Essential Steps for FDA Complaint Handling Medical Device What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. In this episode, lisa van. What Is Medical Device Complaints.
From www.youtube.com
Medical Device Complaint Handling YouTube What Is Medical Device Complaints Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. What Is Medical Device Complaints.
From www.slideserve.com
PPT Medical Device Complaint Management Market PowerPoint What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the. What Is Medical Device Complaints.
From www.slideserve.com
PPT Medical Device Complaint Management Market PowerPoint What Is Medical Device Complaints No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and.. What Is Medical Device Complaints.
From www.youtube.com
Complaint Handling and Medical Device Reporting YouTube What Is Medical Device Complaints The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation. What Is Medical Device Complaints.
From www.slideserve.com
PPT Medical Device Complaint Management Market PowerPoint What Is Medical Device Complaints Yet, complaint handling in the device industry involves so much more than placating upset customers. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr).. What Is Medical Device Complaints.
From www.practicebuilders.com
The ‘Secret’ to Handling Patient Complaints Blog What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device complaint handling process is a systematic approach employed. What Is Medical Device Complaints.
From www.vrogue.co
The Fundamentals Of Medical Device Complaint Handling vrogue.co What Is Medical Device Complaints Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. In this episode, lisa van ryn, a seasoned. What Is Medical Device Complaints.
From www.scribd.com
FDA Medical Device Complaint Form What Is Medical Device Complaints No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). Yet, complaint handling in the device industry involves so much more than placating upset customers.. What Is Medical Device Complaints.
From issuu.com
Medical device complaint handling by John What Is Medical Device Complaints In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in. What Is Medical Device Complaints.
From www.pdffiller.com
Fillable Online MEDICAL DEVICE COMPLAINT/ INCIDENT FORM Fax Email Print What Is Medical Device Complaints The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating upset customers. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). No matter the risk level of. What Is Medical Device Complaints.
From www.qualitymeddev.com
Complaint Handling Process for Medical Device Manufacturers What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Yet, complaint handling in the. What Is Medical Device Complaints.
From www.slideserve.com
PPT Medical Device Complaint Management Market PowerPoint What Is Medical Device Complaints Yet, complaint handling in the device industry involves so much more than placating upset customers. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation. What Is Medical Device Complaints.
From www.regdesk.co
MDA on Complaint Handling and Problem Reporting What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry involves so much more than placating upset customers. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). 21 cfr 820.3(b) any written,. What Is Medical Device Complaints.
From medtechintelligence.com
The Integration of Complaint Handling and Risk Management MedTech What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the. What Is Medical Device Complaints.
From www.slideserve.com
PPT Medical Device Complaint Management Market PowerPoint What Is Medical Device Complaints No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. 21 cfr 820.3(b) any written, electronic,. What Is Medical Device Complaints.
From chartexamples.com
Medical Device Complaint Handling Flowchart Chart Examples What Is Medical Device Complaints No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21. What Is Medical Device Complaints.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Complaints and 21 CFR 803 What Is Medical Device Complaints In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device reporting (mdr). What Is Medical Device Complaints.
From www.linkedin.com
How to Address Complaints with the Medical Device Industry What Is Medical Device Complaints 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device. What Is Medical Device Complaints.
From 365505.com
Medical Device Complaint Handling Process / Complaint Management What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. In this episode, lisa van ryn, a seasoned expert from greenlight guru,. What Is Medical Device Complaints.
From www.defectmaestro.com
5 Steps to Streamline Your Medical Device Complaint Handling Process What Is Medical Device Complaints 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting. What Is Medical Device Complaints.
From www.simplerqms.com
Medical Device Complaint Handling Processes and Best Practices What Is Medical Device Complaints 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). Yet, complaint handling in the device industry involves so much more than placating upset customers. The. What Is Medical Device Complaints.
From www.greenlight.guru
Why Poor Design Controls Mean More Medical Device Complaints What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply. What Is Medical Device Complaints.
From www.greenlight.guru
Medical Device Complaint Handling Procedure [+Flowchart] What Is Medical Device Complaints No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on. What Is Medical Device Complaints.
From www.simplerqms.com
Medical Device Complaint Handling Process What Is Medical Device Complaints The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating upset customers. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. In this episode, lisa van ryn, a. What Is Medical Device Complaints.
From www.greenlight.guru
Medical Device Complaint Handling Flow Chart [Free Download] What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. No matter the risk level of your medical device, in order. What Is Medical Device Complaints.
From www.simplerqms.com
Medical Device Complaint Handling Process What Is Medical Device Complaints The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,.. What Is Medical Device Complaints.
From deviceevents.com
Medical Device Complaints How Device Events Empower Device Events What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed. What Is Medical Device Complaints.
From www.greenlight.guru
Free Complaint Template for Medical Devices What Is Medical Device Complaints The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,.. What Is Medical Device Complaints.
From www.youtube.com
Secrets of Medical Device Complaint Management Short YouTube What Is Medical Device Complaints No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry involves so much more than placating upset customers. In this episode, lisa van ryn,. What Is Medical Device Complaints.
From www.greenlight.guru
How To Reduce & Prevent Medical Device Complaints What Is Medical Device Complaints No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and.. What Is Medical Device Complaints.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Complaints and 21 CFR 803 What Is Medical Device Complaints The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. What Is Medical Device Complaints.
From www.slideshare.net
Medical device complaint management market What Is Medical Device Complaints In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. No matter the risk level of your medical device, in order to be an iso 13485. What Is Medical Device Complaints.
From issuu.com
Medical Device Complaint Handling Training by Medical Device GMP What Is Medical Device Complaints 21 cfr 820.3(b) any written, electronic, or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness,. In this episode, lisa van ryn, a seasoned expert from greenlight guru, shares her extensive knowledge on the fda’s medical device reporting (mdr). No matter the risk level of your medical device, in order to be an iso 13485. What Is Medical Device Complaints.