Antacid Reaction With Water . Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Water and naoh solutions can absorb carbon dioxide, co2, gas from the air, which will react with water to form carbonic acid, h2co3. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. What is important about equation 2 above is that before antacids. Conversely, strong bases react completely. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Antacids are weak bases, and the goal is to measure how much acid can be neutralized by a particular antacid (in other words, you will.
from www.numerade.com
What is important about equation 2 above is that before antacids. Water and naoh solutions can absorb carbon dioxide, co2, gas from the air, which will react with water to form carbonic acid, h2co3. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. Conversely, strong bases react completely. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Antacids are weak bases, and the goal is to measure how much acid can be neutralized by a particular antacid (in other words, you will. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water.
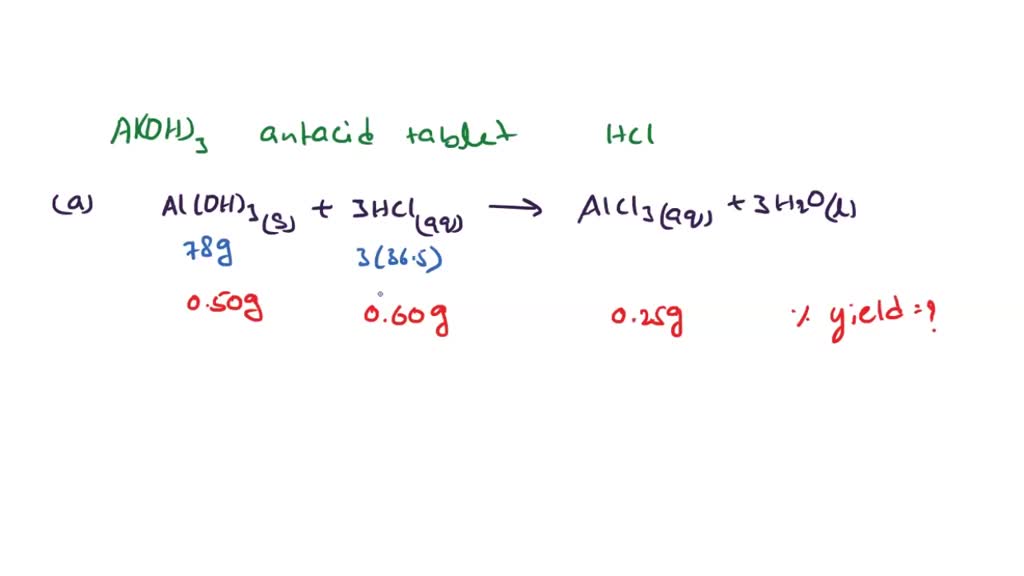
SOLVED Aluminum hydroxide in antacid tablets neutralize hydrochloric
Antacid Reaction With Water Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Conversely, strong bases react completely. What is important about equation 2 above is that before antacids. Antacids are weak bases, and the goal is to measure how much acid can be neutralized by a particular antacid (in other words, you will. Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Water and naoh solutions can absorb carbon dioxide, co2, gas from the air, which will react with water to form carbonic acid, h2co3. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water.
From www.numerade.com
SOLVEDAntacid Fizz When an antacid tablet dissolves in water, the fizz Antacid Reaction With Water Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Conversely, strong bases react completely. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Antacids are. Antacid Reaction With Water.
From www.thoughtco.com
Dissociation Reaction Definition and Examples Antacid Reaction With Water Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. The formulation of aluminum hydrochloride. Antacid Reaction With Water.
From www.youtube.com
Neutralization Reaction of an Antacid YouTube Antacid Reaction With Water Conversely, strong bases react completely. Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially. Antacid Reaction With Water.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID1994845 Antacid Reaction With Water Antacids are weak bases, and the goal is to measure how much acid can be neutralized by a particular antacid (in other words, you will. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water.. Antacid Reaction With Water.
From www.numerade.com
Antacid Fizz When an antacid tablet dissolves in water, the fizz is due Antacid Reaction With Water Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. The formulation of aluminum hydrochloride and water results in the neutralization of the acid. Antacid Reaction With Water.
From chem.libretexts.org
4.3 AcidBase Reactions Chemistry LibreTexts Antacid Reaction With Water Water and naoh solutions can absorb carbon dioxide, co2, gas from the air, which will react with water to form carbonic acid, h2co3. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. The formulation of aluminum hydrochloride and water results in the neutralization of the. Antacid Reaction With Water.
From www.slideshare.net
Antacids Antacid Reaction With Water Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride. Antacid Reaction With Water.
From brainly.com
When an antacid tablet dissolves in water, the fizz is due to a Antacid Reaction With Water Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Conversely, strong bases react completely. Water and naoh solutions can absorb carbon dioxide, co2, gas from the air, which will react with water to form carbonic. Antacid Reaction With Water.
From www.numerade.com
SOLVED this experiment; you will be reacting commerciallyavailable Antacid Reaction With Water Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Conversely, strong bases react completely. The formulation of aluminum hydrochloride and water results in. Antacid Reaction With Water.
From slideplayer.com
Chapter 1 Chemistry and Measurements ppt download Antacid Reaction With Water Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. What is important about equation 2 above is. Antacid Reaction With Water.
From fphoto.photoshelter.com
science chemistry acid base antacid Fundamental Photographs The Art Antacid Reaction With Water Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Water and naoh solutions can absorb carbon dioxide, co2, gas from the air, which will react with water to form carbonic acid, h2co3.. Antacid Reaction With Water.
From www.numerade.com
When an antacid tablet dissolves in water, the fizz is due to a Antacid Reaction With Water Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. Antacids are weak bases, and the goal is to measure how much acid can be neutralized by a particular antacid (in other words, you will. Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining. Antacid Reaction With Water.
From www.youtube.com
Antacid reaction forming carbonated water YouTube Antacid Reaction With Water The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Conversely, strong bases react completely. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach. Antacid Reaction With Water.
From www.sciencephoto.com
Antacid tablet dissolving in glass of water Stock Image M635/0011 Antacid Reaction With Water Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. What is important about equation 2 above is that before antacids. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Effervescent tablets are tablets which are designed to. Antacid Reaction With Water.
From www.numerade.com
SOLVED Data Table 2 Carbon Dioxide Reaction Observations Reaction Antacid Reaction With Water Conversely, strong bases react completely. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Effervescent tablets are. Antacid Reaction With Water.
From www.youtube.com
Experiment Back Titration (antacid) YouTube Antacid Reaction With Water Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride. Antacid Reaction With Water.
From www.numerade.com
SOLVED Antacid; Milk of Magnesia suspension of magnesium hydroxide, Mg Antacid Reaction With Water Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Equation. Antacid Reaction With Water.
From www.slideshare.net
Antacids Antacid Reaction With Water Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Antacids are weak bases,. Antacid Reaction With Water.
From www.flinnsci.ca
Neutralization Reaction of an Antacid Flinn Scientific Antacid Reaction With Water What is important about equation 2 above is that before antacids. Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the. Antacid Reaction With Water.
From www.youtube.com
D.4 Antacids (SL) YouTube Antacid Reaction With Water Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Conversely, strong bases react completely. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride,. Antacid Reaction With Water.
From www.slideserve.com
PPT Antacids PowerPoint Presentation ID1994845 Antacid Reaction With Water Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. The formulation of aluminum hydrochloride and. Antacid Reaction With Water.
From www.youtube.com
3 Antacid Tablet Reaction in Hot & Cold Water YouTube Antacid Reaction With Water What is important about equation 2 above is that before antacids. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Antacids are weak bases, and the goal is to measure how much acid can be neutralized. Antacid Reaction With Water.
From slideplayer.com
Acids and Bases. ppt download Antacid Reaction With Water Conversely, strong bases react completely. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Antacids are weak bases, and the goal is to measure how much acid can be neutralized by a particular antacid (in other words, you will. Equation. Antacid Reaction With Water.
From sites.google.com
Neutralization MissReyes8thgradeScience Antacid Reaction With Water Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. What is important about equation 2 above is that before antacids. Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. Effervescent tablets are tablets which are designed to. Antacid Reaction With Water.
From www.sciencephoto.com
Antacid reacts with hydrochloric acid Stock Image C028/0756 Antacid Reaction With Water The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Strong acids react completely. Antacid Reaction With Water.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID3824036 Antacid Reaction With Water Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Water. Antacid Reaction With Water.
From slideplayer.com
Chapter 8 Chemical Reactions ppt download Antacid Reaction With Water Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon. Antacid Reaction With Water.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID1781850 Antacid Reaction With Water Conversely, strong bases react completely. Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. Water and naoh solutions can absorb carbon dioxide, co2, gas from the air, which will react with water to form. Antacid Reaction With Water.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID4271901 Antacid Reaction With Water Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Water and naoh solutions can absorb carbon dioxide,. Antacid Reaction With Water.
From www.sciencephoto.com
Antacid Reacting with Acid Stock Image C028/1224 Science Photo Antacid Reaction With Water Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Water and naoh solutions can absorb carbon dioxide, co2, gas from the air, which will react with water to form carbonic acid, h2co3.. Antacid Reaction With Water.
From www.numerade.com
SOLVED Aluminum hydroxide in antacid tablets neutralize hydrochloric Antacid Reaction With Water Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Conversely, strong bases react completely. Hydrochloric acid (hcl) is one of the substances found. Antacid Reaction With Water.
From www.youtube.com
2 Whole Antacid Tablet vs. Crushed Antacid Tablet reaction with Antacid Reaction With Water What is important about equation 2 above is that before antacids. Sodium bicarbonate, a rapidly acting antacid, reacts rapidly with gastric hcl in the stomach to produce sodium chloride, carbon dioxide, and water. The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Equation 2 says that when hcl and caco₃ react together,. Antacid Reaction With Water.
From www.youtube.com
antacid in water YouTube Antacid Reaction With Water Conversely, strong bases react completely. The formulation of aluminum hydrochloride and water results in the neutralization of the acid in the stomach. Antacids are weak bases, and the goal is to measure how much acid can be neutralized by a particular antacid (in other words, you will. Equation 2 says that when hcl and caco₃ react together, they produce calcium. Antacid Reaction With Water.
From www.scribd.com
antacid.ppt Acid Properties Of Water Antacid Reaction With Water Effervescent tablets are tablets which are designed to dissolve in water, and then release carbon dioxide. Hydrochloric acid (hcl) is one of the substances found in gastric juices secreted by the lining of. Equation 2 says that when hcl and caco₃ react together, they produce calcium chloride (cacl₂), carbon dioxide, and water. Strong acids react completely with water to produce. Antacid Reaction With Water.
From www.numerade.com
SOLVED Data Table 2 Carbon Dioxide Reaction Observations Reaction Antacid Reaction With Water Excess bicarbonate rapidly empties into the small intestine, where it is then absorbed. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Antacids are weak bases, and the goal is to measure how much acid can be neutralized by a particular antacid (in other words,. Antacid Reaction With Water.