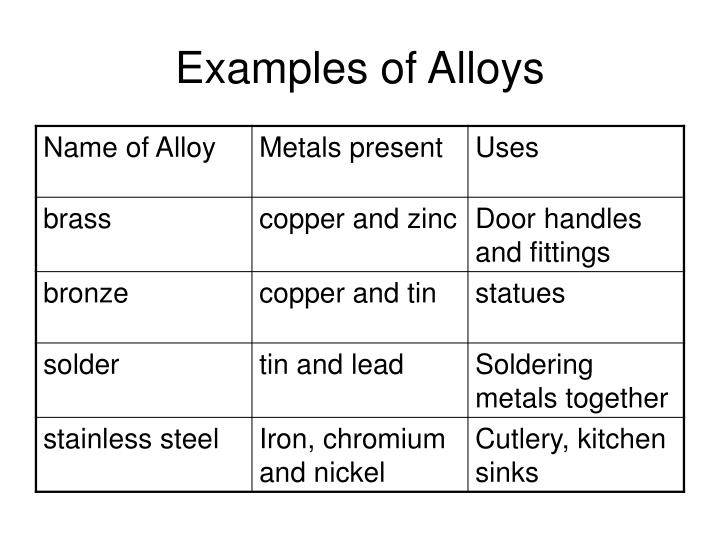
Metal Alloy Definition . Comparing properties of alloys and pure metals. A combination of iron (metal) and. Many alloys are mixtures of two or more metals. An alloy is a substance made by combining together two or more elements where the primary element is a metal. Most alloys form by melting the elements together. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. The components of alloys are. For example, brass is an. Alloys can be formed by substituting one metal atom. An alloy is a substance made by melting two or more elements together, at least one of them metal. An alloy crystallizes upon cooling into a solid solution, mixture, or. Many pure metals are too soft for many uses. They can be made harder by adding.
from www.slideserve.com
An alloy is a substance made by melting two or more elements together, at least one of them metal. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Many alloys are mixtures of two or more metals. Many pure metals are too soft for many uses. Alloys can be formed by substituting one metal atom. An alloy crystallizes upon cooling into a solid solution, mixture, or. Most alloys form by melting the elements together. A combination of iron (metal) and. They can be made harder by adding. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements.
PPT Physical Properties of Metals PowerPoint Presentation ID5519685
Metal Alloy Definition Many pure metals are too soft for many uses. An alloy is a substance made by combining together two or more elements where the primary element is a metal. Comparing properties of alloys and pure metals. Many pure metals are too soft for many uses. A combination of iron (metal) and. An alloy crystallizes upon cooling into a solid solution, mixture, or. For example, brass is an. Many alloys are mixtures of two or more metals. Most alloys form by melting the elements together. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Alloys can be formed by substituting one metal atom. They can be made harder by adding. An alloy is a substance made by melting two or more elements together, at least one of them metal. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. The components of alloys are.
From www.mechical.com
Ferrous Metal Definition, Types, Uses, Properties Metal Alloy Definition Alloy, metallic substance composed of two or more elements, as either a compound or a solution. An alloy is a substance made by combining together two or more elements where the primary element is a metal. Alloys can be formed by substituting one metal atom. The components of alloys are. A combination of iron (metal) and. Comparing properties of alloys. Metal Alloy Definition.
From sciencenotes.org
What Is an Alloy? Definition and Examples Metal Alloy Definition Most alloys form by melting the elements together. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Comparing properties of alloys and pure metals. Many alloys are mixtures of two or more metals. A combination of iron (metal) and. They can be made harder by adding. Many pure metals are. Metal Alloy Definition.
From byjus.com
Alloy Formation In Transition Metals Alloy Of Copper And Zinc Metal Alloy Definition Alloys can be formed by substituting one metal atom. An alloy crystallizes upon cooling into a solid solution, mixture, or. A combination of iron (metal) and. The components of alloys are. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. For example, brass is an. An alloy is a mixture of metals that. Metal Alloy Definition.
From gamesmartz.com
Alloy Definition Easy to Understand GameSmartz Metal Alloy Definition An alloy is a substance made by melting two or more elements together, at least one of them metal. The components of alloys are. An alloy is a substance made by combining together two or more elements where the primary element is a metal. Alloy, metallic substance composed of two or more elements, as either a compound or a solution.. Metal Alloy Definition.
From www.pinterest.com
What Is an Alloy? Definition and Examples Chemistry, Alloy, Materials Metal Alloy Definition Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Alloys can be formed by substituting one metal atom. For example, brass is an. The components of alloys are. Many pure metals are too soft for many uses. Most alloys form by melting the elements together. They can be made harder by adding. An. Metal Alloy Definition.
From scienceinfo.com
Alloys Characteristics, Classification, Types, Benefits, Limitations Metal Alloy Definition An alloy crystallizes upon cooling into a solid solution, mixture, or. An alloy is a substance made by melting two or more elements together, at least one of them metal. Alloys can be formed by substituting one metal atom. An alloy is a substance made by combining together two or more elements where the primary element is a metal. An. Metal Alloy Definition.
From www.slideshare.net
alloys and their properties Metal Alloy Definition They can be made harder by adding. Many alloys are mixtures of two or more metals. Comparing properties of alloys and pure metals. For example, brass is an. An alloy crystallizes upon cooling into a solid solution, mixture, or. Most alloys form by melting the elements together. Many pure metals are too soft for many uses. An alloy is a. Metal Alloy Definition.
From www.techglads.com
Properties of alloys in Engineering Chemistry Tech Glads Metal Alloy Definition An alloy is a substance made by combining together two or more elements where the primary element is a metal. A combination of iron (metal) and. Alloys can be formed by substituting one metal atom. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Many alloys are mixtures of two or more metals.. Metal Alloy Definition.
From www.worksheetsplanet.com
What is a Metal Definition of Metal Elements Metal Alloy Definition Most alloys form by melting the elements together. For example, brass is an. An alloy is a substance made by combining together two or more elements where the primary element is a metal. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Comparing properties of alloys and pure metals. Many alloys are mixtures. Metal Alloy Definition.
From tampasteel.com
What Is a Metal Alloy? Metal vs Metal Alloy Difference Tampa Steel Metal Alloy Definition Alloys can be formed by substituting one metal atom. Most alloys form by melting the elements together. A combination of iron (metal) and. Many alloys are mixtures of two or more metals. An alloy crystallizes upon cooling into a solid solution, mixture, or. Many pure metals are too soft for many uses. An alloy is a substance made by melting. Metal Alloy Definition.
From engineeringlearner.com
Types of Metals and Their Uses [with Pictures] Engineering Learner Metal Alloy Definition An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. An alloy is a substance made by melting two or more elements together, at least one of them metal. For example, brass is an. Most alloys form by melting the elements together. They can be made harder by adding. An alloy. Metal Alloy Definition.
From www.slideserve.com
PPT Physical Properties of Metals PowerPoint Presentation ID5519685 Metal Alloy Definition An alloy is a substance made by melting two or more elements together, at least one of them metal. Most alloys form by melting the elements together. The components of alloys are. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. An alloy is a substance made by combining together. Metal Alloy Definition.
From www.tec-science.com
Typs of alloys tecscience Metal Alloy Definition Comparing properties of alloys and pure metals. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Many alloys are mixtures of two or more metals. For example, brass is an. An alloy crystallizes upon cooling into a solid solution, mixture, or. The components of alloys are. A combination of iron. Metal Alloy Definition.
From www.iqsdirectory.com
Metal Alloys Principles, Types, Advantages and Applications Metal Alloy Definition Many alloys are mixtures of two or more metals. For example, brass is an. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Comparing properties of alloys and pure metals. An alloy crystallizes upon cooling into a solid solution, mixture, or. They can be made harder by adding. An alloy is a substance. Metal Alloy Definition.
From pre.unionfab.com
Alloy Steel vs Stainless Steel vs Steel An Ultimate Comparison Metal Alloy Definition The components of alloys are. Many pure metals are too soft for many uses. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. They can be made harder by adding. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Alloys can be formed. Metal Alloy Definition.
From www.iqsdirectory.com
Metal Alloys Principles, Types, Advantages and Applications Metal Alloy Definition Most alloys form by melting the elements together. Many alloys are mixtures of two or more metals. Alloys can be formed by substituting one metal atom. A combination of iron (metal) and. For example, brass is an. Many pure metals are too soft for many uses. They can be made harder by adding. The components of alloys are. An alloy. Metal Alloy Definition.
From msestudent.com
What are Alloys? (Definition, Examples, and Metallurgy) Materials Metal Alloy Definition An alloy crystallizes upon cooling into a solid solution, mixture, or. An alloy is a substance made by melting two or more elements together, at least one of them metal. Comparing properties of alloys and pure metals. For example, brass is an. They can be made harder by adding. Alloy, metallic substance composed of two or more elements, as either. Metal Alloy Definition.
From www.slideserve.com
PPT Metal alloys PowerPoint Presentation, free download ID5703763 Metal Alloy Definition Most alloys form by melting the elements together. They can be made harder by adding. Alloys can be formed by substituting one metal atom. An alloy is a substance made by melting two or more elements together, at least one of them metal. Many alloys are mixtures of two or more metals. For example, brass is an. Comparing properties of. Metal Alloy Definition.
From www.iqsdirectory.com
Metal Alloys Principles, Types, Advantages and Applications Metal Alloy Definition Comparing properties of alloys and pure metals. Alloys can be formed by substituting one metal atom. An alloy is a substance made by combining together two or more elements where the primary element is a metal. An alloy crystallizes upon cooling into a solid solution, mixture, or. For example, brass is an. An alloy is a mixture of metals that. Metal Alloy Definition.
From www.expii.com
Alloys — Overview & Examples Expii Metal Alloy Definition Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Many pure metals are too soft for many uses. An alloy is a substance made by combining together two or more elements where the primary element is a metal. Most alloys form by melting the elements together. Alloys can be formed by substituting one. Metal Alloy Definition.
From www.animalia-life.club
Metal Alloys Metal Alloy Definition Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Many alloys are mixtures of two or more metals. Most alloys form by melting the elements together. Many pure metals are too soft for many uses. Comparing properties of alloys and pure metals. An alloy is a substance made by combining together two or. Metal Alloy Definition.
From sciencenotes.org
What Are Noble Metals? Definition and List Metal Alloy Definition Alloys can be formed by substituting one metal atom. Many pure metals are too soft for many uses. For example, brass is an. An alloy crystallizes upon cooling into a solid solution, mixture, or. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Comparing properties of alloys and pure metals. A combination of. Metal Alloy Definition.
From www.tes.com
Alloys Edexcel 91 Separate (Triple) Science Teaching Resources Metal Alloy Definition Comparing properties of alloys and pure metals. They can be made harder by adding. Most alloys form by melting the elements together. The components of alloys are. Alloys can be formed by substituting one metal atom. Many pure metals are too soft for many uses. A combination of iron (metal) and. An alloy is a substance made by combining together. Metal Alloy Definition.
From www.thoughtco.com
Properties, Composition, and Production of Metal Alloys Metal Alloy Definition Comparing properties of alloys and pure metals. For example, brass is an. Many pure metals are too soft for many uses. An alloy is a substance made by melting two or more elements together, at least one of them metal. Many alloys are mixtures of two or more metals. They can be made harder by adding. Alloy, metallic substance composed. Metal Alloy Definition.
From dz6wltn9e7i51.cloudfront.net
Alloys Definition, Composition, Types, Properties, and Applications Metal Alloy Definition A combination of iron (metal) and. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Most alloys form by melting the elements together. The components of alloys are. Many pure metals are too soft for many uses. An alloy is a substance made by melting two or more elements together,. Metal Alloy Definition.
From www.visualcapitalist.com
Infographic 20 Common Metal Alloys and What They're Made Of Metal Alloy Definition An alloy is a substance made by combining together two or more elements where the primary element is a metal. They can be made harder by adding. An alloy is a substance made by melting two or more elements together, at least one of them metal. For example, brass is an. An alloy is a mixture of metals that has. Metal Alloy Definition.
From animalia-life.club
Brass Alloy Composition Metal Alloy Definition An alloy crystallizes upon cooling into a solid solution, mixture, or. Alloys can be formed by substituting one metal atom. An alloy is a substance made by combining together two or more elements where the primary element is a metal. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. For. Metal Alloy Definition.
From stock.adobe.com
Alloy vs pure metal comparison with iron and steel properties outline Metal Alloy Definition Alloy, metallic substance composed of two or more elements, as either a compound or a solution. An alloy crystallizes upon cooling into a solid solution, mixture, or. The components of alloys are. They can be made harder by adding. Most alloys form by melting the elements together. An alloy is a mixture of metals that has bulk metallic properties different. Metal Alloy Definition.
From scienceinfo.com
Alloys Characteristics, Classification, Types, Benefits, Limitations Metal Alloy Definition An alloy crystallizes upon cooling into a solid solution, mixture, or. The components of alloys are. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. They can be made harder by adding. Comparing properties of alloys and pure metals. An alloy is a substance made by combining together two or. Metal Alloy Definition.
From www.animalia-life.club
Alloys Chemistry Metal Alloy Definition Most alloys form by melting the elements together. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Many pure metals are too soft for many uses. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. For example, brass is an. The components of. Metal Alloy Definition.
From blog.thepipingmart.com
Aluminium Alloys Types, Properties and Uses Metal Alloy Definition For example, brass is an. They can be made harder by adding. Many alloys are mixtures of two or more metals. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Many pure metals are. Metal Alloy Definition.
From www.mechanicaleducation.com
Alloy Steel And Different Types Of Alloy Steel Mechanical Education Metal Alloy Definition Most alloys form by melting the elements together. An alloy crystallizes upon cooling into a solid solution, mixture, or. Alloys can be formed by substituting one metal atom. Many alloys are mixtures of two or more metals. The components of alloys are. A combination of iron (metal) and. An alloy is a substance made by combining together two or more. Metal Alloy Definition.
From msestudent.com
What are Alloys? (Definition, Examples, and Metallurgy) Materials Metal Alloy Definition Comparing properties of alloys and pure metals. Many alloys are mixtures of two or more metals. An alloy is a substance made by combining together two or more elements where the primary element is a metal. They can be made harder by adding. The components of alloys are. Alloys can be formed by substituting one metal atom. An alloy is. Metal Alloy Definition.
From www.slideserve.com
PPT METAL ALLOYS (Homogeneous Mixtures!) PowerPoint Presentation Metal Alloy Definition A combination of iron (metal) and. The components of alloys are. They can be made harder by adding. An alloy is a substance made by melting two or more elements together, at least one of them metal. Comparing properties of alloys and pure metals. An alloy is a mixture of metals that has bulk metallic properties different from those of. Metal Alloy Definition.
From ar.inspiredpencil.com
Metal Alloys Examples Metal Alloy Definition An alloy crystallizes upon cooling into a solid solution, mixture, or. They can be made harder by adding. An alloy is a mixture of metals that has bulk metallic properties different from those of its constituent elements. Most alloys form by melting the elements together. Comparing properties of alloys and pure metals. An alloy is a substance made by melting. Metal Alloy Definition.