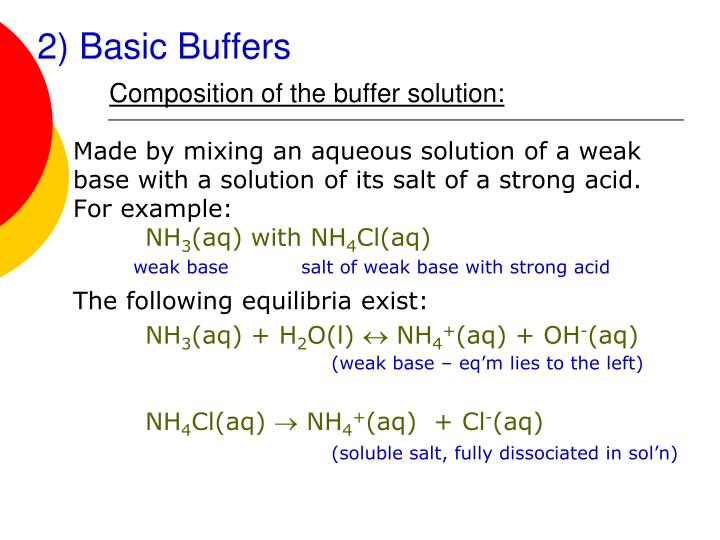
Buffer Solution Equilibrium . Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. By removal of h+(aq)ions we. systematic solution to buffer problems. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. how it works. the mechanism involves a buffer, a solution that resists dramatic changes in ph.
from www.slideserve.com
A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. By removal of h+(aq)ions we. systematic solution to buffer problems. how it works. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3.
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
Buffer Solution Equilibrium Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. systematic solution to buffer problems. By removal of h+(aq)ions we. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. how it works. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed.
From www.studypool.com
SOLUTION The equilibrium and Buffer Studypool Buffer Solution Equilibrium the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. By removal of h+(aq)ions we. systematic solution to buffer problems. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that can resist ph. Buffer Solution Equilibrium.
From www.slideserve.com
PPT Hydrolysis of Salts and pH of Buffer Solutions PowerPoint Buffer Solution Equilibrium a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. By removal of h+(aq)ions we. Equation 6.8.2 is written in terms of the equilibrium concentrations. Buffer Solution Equilibrium.
From www.studypool.com
SOLUTION Chemical equilibrium buffer solution calculations Studypool Buffer Solution Equilibrium how it works. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. systematic solution to buffer problems. a buffer is a solution. Buffer Solution Equilibrium.
From www.youtube.com
Ionic Equilibrium Buffer Solution Chemistry Allen Digital YouTube Buffer Solution Equilibrium a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. systematic solution to buffer problems. By removal of h+(aq)ions we. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist. Buffer Solution Equilibrium.
From www.studypool.com
SOLUTION The equilibrium and Buffer Studypool Buffer Solution Equilibrium systematic solution to buffer problems. By removal of h+(aq)ions we. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. the mechanism involves a buffer, a solution that resists dramatic changes in ph. how. Buffer Solution Equilibrium.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Equilibrium By removal of h+(aq)ions we. how it works. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. the mechanism involves a buffer, a solution that resists dramatic changes. Buffer Solution Equilibrium.
From courses.lumenlearning.com
Buffers Chemistry for Majors Buffer Solution Equilibrium the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. systematic solution to buffer problems. how it works. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. a buffer is a solution. Buffer Solution Equilibrium.
From www.chegg.com
Solved Determine the pH of a buffer solution by constructing Buffer Solution Equilibrium the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. how it works. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution contains both a weak acid and a weak. Buffer Solution Equilibrium.
From studylib.net
Chapter 1 Fundamental Concepts Buffer Solution Equilibrium how it works. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. systematic solution to buffer problems. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. By removal of h+(aq)ions we. the mechanism involves a buffer, a solution that resists dramatic changes. Buffer Solution Equilibrium.
From www.studypool.com
SOLUTION Chemical equilibrium with examples reaction explanation Buffer Solution Equilibrium A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. By removal of h+(aq)ions we. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that can resist. Buffer Solution Equilibrium.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content Buffer Solution Equilibrium the mechanism involves a buffer, a solution that resists dramatic changes in ph. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. By removal of h+(aq)ions we. how it. Buffer Solution Equilibrium.
From www.youtube.com
Preparation and Properties of Buffer Solution Chemical Equilibrium Buffer Solution Equilibrium the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. how it works. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are. Buffer Solution Equilibrium.
From www.slideserve.com
PPT Chemical equilibria, principle of pH, buffers PowerPoint Buffer Solution Equilibrium systematic solution to buffer problems. how it works. By removal of h+(aq)ions we. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the purpose of a buffer system is to keep the. Buffer Solution Equilibrium.
From www.slideserve.com
PPT CHEMISTRY Chapter 15 Applications of Aqueous Equilibria Buffer Solution Equilibrium the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. how it works. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are. Buffer Solution Equilibrium.
From www.studocu.com
Equilibrium Chemistry Buffer Solutions 1 C Equilibrium Chemistry Buffer Solution Equilibrium a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. By removal of h+(aq)ions we. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. systematic solution to buffer problems. the mechanism involves a buffer, a solution that resists. Buffer Solution Equilibrium.
From www.youtube.com
Buffer solutionTypes of buffer solutionPh of buffer solution Buffer Solution Equilibrium a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. how it works. systematic solution to buffer problems. A buffer solution contains both. Buffer Solution Equilibrium.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer Solution Equilibrium Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. A buffer. Buffer Solution Equilibrium.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution Equilibrium how it works. systematic solution to buffer problems. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. By removal of h+(aq)ions we.. Buffer Solution Equilibrium.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Equilibrium Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. By removal of h+(aq)ions we. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the purpose of a buffer system is. Buffer Solution Equilibrium.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer Solution Equilibrium the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. a buffer is a solution that can resist ph change upon the addition of an. Buffer Solution Equilibrium.
From www.slideserve.com
PPT Chapter 16 Aqueous Ionic Equilibrium PowerPoint Presentation Buffer Solution Equilibrium how it works. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. By removal of h+(aq)ions we. systematic solution to buffer problems. a buffer is a solution that. Buffer Solution Equilibrium.
From www.scribd.com
Experiment 7 Acid Base Equilibrium and Buffers PDF Buffer Solution Buffer Solution Equilibrium systematic solution to buffer problems. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. how it works. Equation 6.8.2 is written in terms of the equilibrium concentrations of. Buffer Solution Equilibrium.
From www.studypool.com
SOLUTION Chemical equilibrium with examples reaction explanation Buffer Solution Equilibrium systematic solution to buffer problems. By removal of h+(aq)ions we. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. the. Buffer Solution Equilibrium.
From www.youtube.com
Aqueous Solution Equilibrium 01 Buffer Solutions YouTube Buffer Solution Equilibrium Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. By removal of h+(aq)ions we. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. the purpose of a buffer system is to. Buffer Solution Equilibrium.
From www.studypool.com
SOLUTION The equilibrium and Buffer Studypool Buffer Solution Equilibrium systematic solution to buffer problems. how it works. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. the mechanism involves a buffer, a solution that resists dramatic changes in ph. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added. Buffer Solution Equilibrium.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Buffer Solution Equilibrium a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. By removal of h+(aq)ions we. systematic solution to buffer problems. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. the purpose of a buffer system is to keep the ph of a solution as. Buffer Solution Equilibrium.
From www.youtube.com
PH of buffer solution MCQ problem on ionic equilibrium YouTube Buffer Solution Equilibrium the mechanism involves a buffer, a solution that resists dramatic changes in ph. By removal of h+(aq)ions we. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions. Buffer Solution Equilibrium.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps Buffer Solution Equilibrium By removal of h+(aq)ions we. the mechanism involves a buffer, a solution that resists dramatic changes in ph. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. a buffer is a solution that can resist ph change upon the addition of an. Buffer Solution Equilibrium.
From www.slideserve.com
PPT Equilibrium Part 2 Chemistry 2 PowerPoint Presentation, free Buffer Solution Equilibrium a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions. Buffer Solution Equilibrium.
From www.slideshare.net
2012 topic 18 2 buffer solutions Buffer Solution Equilibrium the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. the mechanism involves a buffer, a solution that resists dramatic changes in ph. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. systematic solution to buffer problems. a. Buffer Solution Equilibrium.
From www.studypool.com
SOLUTION Chemical equilibrium buffer solution calculations Studypool Buffer Solution Equilibrium systematic solution to buffer problems. how it works. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. By removal of h+(aq)ions we. the purpose of a buffer. Buffer Solution Equilibrium.
From wisc.pb.unizin.org
Day 36 Buffer Solutions Chemistry 109, Fall 2020 Buffer Solution Equilibrium systematic solution to buffer problems. By removal of h+(aq)ions we. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. how it works. Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. the purpose of a buffer system is to keep the ph. Buffer Solution Equilibrium.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Buffer Solution Equilibrium By removal of h+(aq)ions we. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. systematic solution to buffer problems. A buffer solution contains both a weak acid and a weak base in equilibrium, which resist changes in ph. how it works. Equation 6.8.2 is written in terms. Buffer Solution Equilibrium.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID Buffer Solution Equilibrium Equation 6.8.2 is written in terms of the equilibrium concentrations of ch 3. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. the. Buffer Solution Equilibrium.
From www.youtube.com
Equilibrium Class 11 Chemistry Buffer Solutions YouTube Buffer Solution Equilibrium a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. systematic solution to buffer problems. the purpose of a buffer system is to keep the ph of a solution as constant as possible if h+(aq)ions are added or removed. Equation 6.8.2 is written in terms of the equilibrium. Buffer Solution Equilibrium.