Euler Equation Ideal Gas . Where is the number of moles of gas, is the molar mass of the gas (i.e. In this lecture we study some properties of the euler equations. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. we use the ideal gas equations, so: For dry air we get , as it. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. A mass of one mole of the gas, e.g. the behavior of ideal compressible gas can be described with euler equations. in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow.
from slidetodoc.com
A mass of one mole of the gas, e.g. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. the behavior of ideal compressible gas can be described with euler equations. In this lecture we study some properties of the euler equations. in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. Where is the number of moles of gas, is the molar mass of the gas (i.e. For dry air we get , as it. we use the ideal gas equations, so:
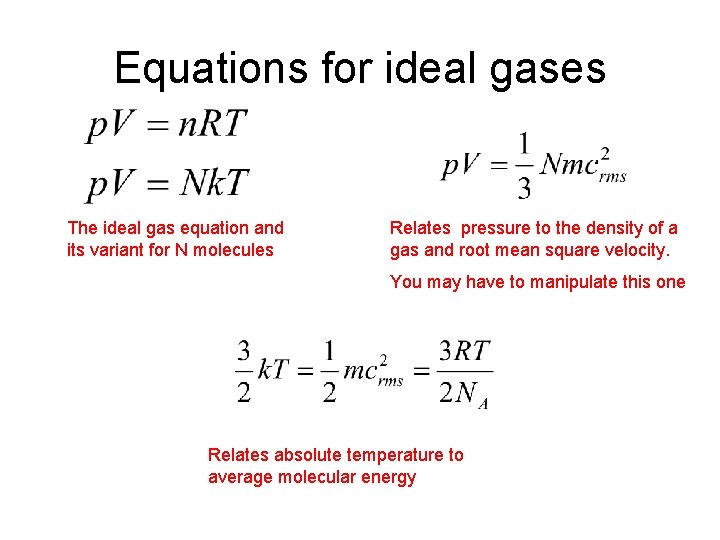
Equations for ideal gases The ideal gas equation
Euler Equation Ideal Gas Where is the number of moles of gas, is the molar mass of the gas (i.e. we use the ideal gas equations, so: in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. For dry air we get , as it. the behavior of ideal compressible gas can be described with euler equations. In this lecture we study some properties of the euler equations. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. A mass of one mole of the gas, e.g. Where is the number of moles of gas, is the molar mass of the gas (i.e. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Euler Equation Ideal Gas A mass of one mole of the gas, e.g. we use the ideal gas equations, so: example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. the ideal gas law is derived from empirical relationships among the pressure, the. Euler Equation Ideal Gas.
From www.slideserve.com
PPT Euler equations of Gas Dynamics PowerPoint Presentation, free Euler Equation Ideal Gas the behavior of ideal compressible gas can be described with euler equations. For dry air we get , as it. Where is the number of moles of gas, is the molar mass of the gas (i.e. In this lecture we study some properties of the euler equations. in this paper we present a classical solution of the isothermal. Euler Equation Ideal Gas.
From www.youtube.com
Euler equation of motion of an ideal fluid Fluid Mechanics YouTube Euler Equation Ideal Gas Where is the number of moles of gas, is the molar mass of the gas (i.e. the behavior of ideal compressible gas can be described with euler equations. in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. example for an ideal gas at temperature t, we. Euler Equation Ideal Gas.
From www.researchgate.net
(PDF) Quasisimple Wave Solutions of Euler’s System of Equations for Euler Equation Ideal Gas in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. In this lecture we study some properties of the euler equations. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. For dry air we get , as. Euler Equation Ideal Gas.
From www.tec-science.com
Derivation of the Euler equation of motion (conservation of momentum Euler Equation Ideal Gas we use the ideal gas equations, so: In this lecture we study some properties of the euler equations. A mass of one mole of the gas, e.g. Where is the number of moles of gas, is the molar mass of the gas (i.e. example for an ideal gas at temperature t, we have pv = nrt, where v. Euler Equation Ideal Gas.
From www.tec-science.com
Derivation of the Euler equation of motion (conservation of momentum Euler Equation Ideal Gas in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. In this lecture we study some properties of the euler equations. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t. Euler Equation Ideal Gas.
From mmerevise.co.uk
The Ideal Gas Equation MME Euler Equation Ideal Gas we use the ideal gas equations, so: Where is the number of moles of gas, is the molar mass of the gas (i.e. the behavior of ideal compressible gas can be described with euler equations. For dry air we get , as it. in this paper we present a classical solution of the isothermal euler equations where. Euler Equation Ideal Gas.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Euler Equation Ideal Gas the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. In this lecture we study some properties of the euler equations. Where is the number of moles of. Euler Equation Ideal Gas.
From www.alamy.com
Ideal gas law, illustration Stock Photo Alamy Euler Equation Ideal Gas the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Where is the number of moles of gas, is the molar mass of the gas (i.e. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of. Euler Equation Ideal Gas.
From slideplayer.com
Research Methods in Acoustics Lecture 5 Reflection and Horn Equation Euler Equation Ideal Gas A mass of one mole of the gas, e.g. the behavior of ideal compressible gas can be described with euler equations. we use the ideal gas equations, so: For dry air we get , as it. Where is the number of moles of gas, is the molar mass of the gas (i.e. the ideal gas law is. Euler Equation Ideal Gas.
From www.youtube.com
Euler's equation Fluid Mechanics YouTube Euler Equation Ideal Gas Where is the number of moles of gas, is the molar mass of the gas (i.e. In this lecture we study some properties of the euler equations. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. For dry air we get , as it. A mass of one. Euler Equation Ideal Gas.
From www.chegg.com
(6.8) Euler. (Thermodynamics, Chemistry) 2 (a) Using Euler Equation Ideal Gas Where is the number of moles of gas, is the molar mass of the gas (i.e. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. in this paper we present a classical solution of the isothermal euler equations where. Euler Equation Ideal Gas.
From www.tokresource.org
Ideal Gas Law and the Euler Relation — TOK Euler Equation Ideal Gas the behavior of ideal compressible gas can be described with euler equations. In this lecture we study some properties of the euler equations. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. A mass of one mole of the. Euler Equation Ideal Gas.
From www.youtube.com
Deriving The Euler Equation YouTube Euler Equation Ideal Gas Where is the number of moles of gas, is the molar mass of the gas (i.e. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. the behavior of ideal compressible gas can be described with euler equations. the. Euler Equation Ideal Gas.
From en.ppt-online.org
The ideal gas equation online presentation Euler Equation Ideal Gas In this lecture we study some properties of the euler equations. A mass of one mole of the gas, e.g. Where is the number of moles of gas, is the molar mass of the gas (i.e. we use the ideal gas equations, so: example for an ideal gas at temperature t, we have pv = nrt, where v. Euler Equation Ideal Gas.
From scienceinfo.com
Ideal Gas Equation Ideal Gas and Laws Euler Equation Ideal Gas we use the ideal gas equations, so: A mass of one mole of the gas, e.g. Where is the number of moles of gas, is the molar mass of the gas (i.e. In this lecture we study some properties of the euler equations. For dry air we get , as it. the ideal gas law is derived from. Euler Equation Ideal Gas.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation ID6652783 Euler Equation Ideal Gas For dry air we get , as it. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. we use the ideal gas equations, so: the behavior of ideal compressible gas can be described with euler equations. In this. Euler Equation Ideal Gas.
From www.tec-science.com
Derivation of the Euler equation of motion (conservation of momentum Euler Equation Ideal Gas the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. In this lecture we study some properties of the euler equations. Where is the number of moles of gas, is the molar mass of the gas (i.e. For dry air we get , as it. in this paper. Euler Equation Ideal Gas.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Euler Equation Ideal Gas example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. In this lecture we study some properties of the euler. Euler Equation Ideal Gas.
From www.tokresource.org
Ideal Gas Law and the Euler Relation — TOK Euler Equation Ideal Gas we use the ideal gas equations, so: the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. Where is. Euler Equation Ideal Gas.
From www.slideserve.com
PPT Euler equations of Gas Dynamics PowerPoint Presentation, free Euler Equation Ideal Gas we use the ideal gas equations, so: the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. In this lecture we study some properties of the euler equations. Where is the number of moles of gas, is the molar mass of the gas (i.e. For dry air we. Euler Equation Ideal Gas.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Euler Equation Ideal Gas the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Where is the number of moles of gas, is the molar mass of the gas (i.e. A mass of one mole of the gas, e.g. the behavior of ideal compressible gas can be described with euler equations. In. Euler Equation Ideal Gas.
From www.britannica.com
Equation of state Definition, Ideal Gas, & Facts Britannica Euler Equation Ideal Gas in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. A mass of one mole of the gas, e.g. we use the ideal gas equations, so: the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Where. Euler Equation Ideal Gas.
From www.grc.nasa.gov
Euler Equations Euler Equation Ideal Gas Where is the number of moles of gas, is the molar mass of the gas (i.e. A mass of one mole of the gas, e.g. the behavior of ideal compressible gas can be described with euler equations. the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. . Euler Equation Ideal Gas.
From slidetodoc.com
Equations for ideal gases The ideal gas equation Euler Equation Ideal Gas Where is the number of moles of gas, is the molar mass of the gas (i.e. in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. For dry air we get , as it. A mass of one mole of the gas, e.g. we use the ideal gas. Euler Equation Ideal Gas.
From www.grc.nasa.gov
Euler Equations Euler Equation Ideal Gas For dry air we get , as it. in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. we use the ideal gas equations, so: A mass of one mole of the gas, e.g. the behavior of ideal compressible gas can be described with euler equations. . Euler Equation Ideal Gas.
From www.chegg.com
Solved A) Euler's hydrodynamic equation of motion for ideal Euler Equation Ideal Gas we use the ideal gas equations, so: in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. the. Euler Equation Ideal Gas.
From byjus.com
Different forms of ideal gas equation Euler Equation Ideal Gas the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. we use the ideal gas equations, so: A mass of one mole of the gas, e.g. in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. In. Euler Equation Ideal Gas.
From www.slideserve.com
PPT CHAPTER 2 PowerPoint Presentation, free download ID1530126 Euler Equation Ideal Gas the behavior of ideal compressible gas can be described with euler equations. we use the ideal gas equations, so: For dry air we get , as it. A mass of one mole of the gas, e.g. In this lecture we study some properties of the euler equations. in this paper we present a classical solution of the. Euler Equation Ideal Gas.
From andymath.com
Euler's Formula Euler Equation Ideal Gas In this lecture we study some properties of the euler equations. For dry air we get , as it. A mass of one mole of the gas, e.g. Where is the number of moles of gas, is the molar mass of the gas (i.e. we use the ideal gas equations, so: in this paper we present a classical. Euler Equation Ideal Gas.
From www.researchgate.net
(PDF) Euler equations for isentropic gas dynamics with general pressure law Euler Equation Ideal Gas the ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. In this lecture we study some properties of the euler equations. we use the ideal gas equations, so: A mass of one mole of the gas, e.g. in this paper we present a classical solution of the. Euler Equation Ideal Gas.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID Euler Equation Ideal Gas in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. the behavior of ideal compressible gas can be described with euler equations. In this lecture we study some properties of the euler equations. A mass of one mole of the gas, e.g. For dry air we get ,. Euler Equation Ideal Gas.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Euler Equation Ideal Gas Where is the number of moles of gas, is the molar mass of the gas (i.e. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. For dry air we get , as it. In this lecture we study some properties. Euler Equation Ideal Gas.
From www.numerade.com
SOLVED The letter e will be recognized as Euler's number. Do not enter Euler Equation Ideal Gas the behavior of ideal compressible gas can be described with euler equations. A mass of one mole of the gas, e.g. example for an ideal gas at temperature t, we have pv = nrt, where v is volume, n is the number of moles, ris contant, and t is. In this lecture we study some properties of the. Euler Equation Ideal Gas.
From www.slideserve.com
PPT Chapter 4 Flowing Fluids & Pressure Variation (part 1 Euler Equation Ideal Gas For dry air we get , as it. Where is the number of moles of gas, is the molar mass of the gas (i.e. in this paper we present a classical solution of the isothermal euler equations where the direction of the gas flow. the ideal gas law is derived from empirical relationships among the pressure, the volume,. Euler Equation Ideal Gas.