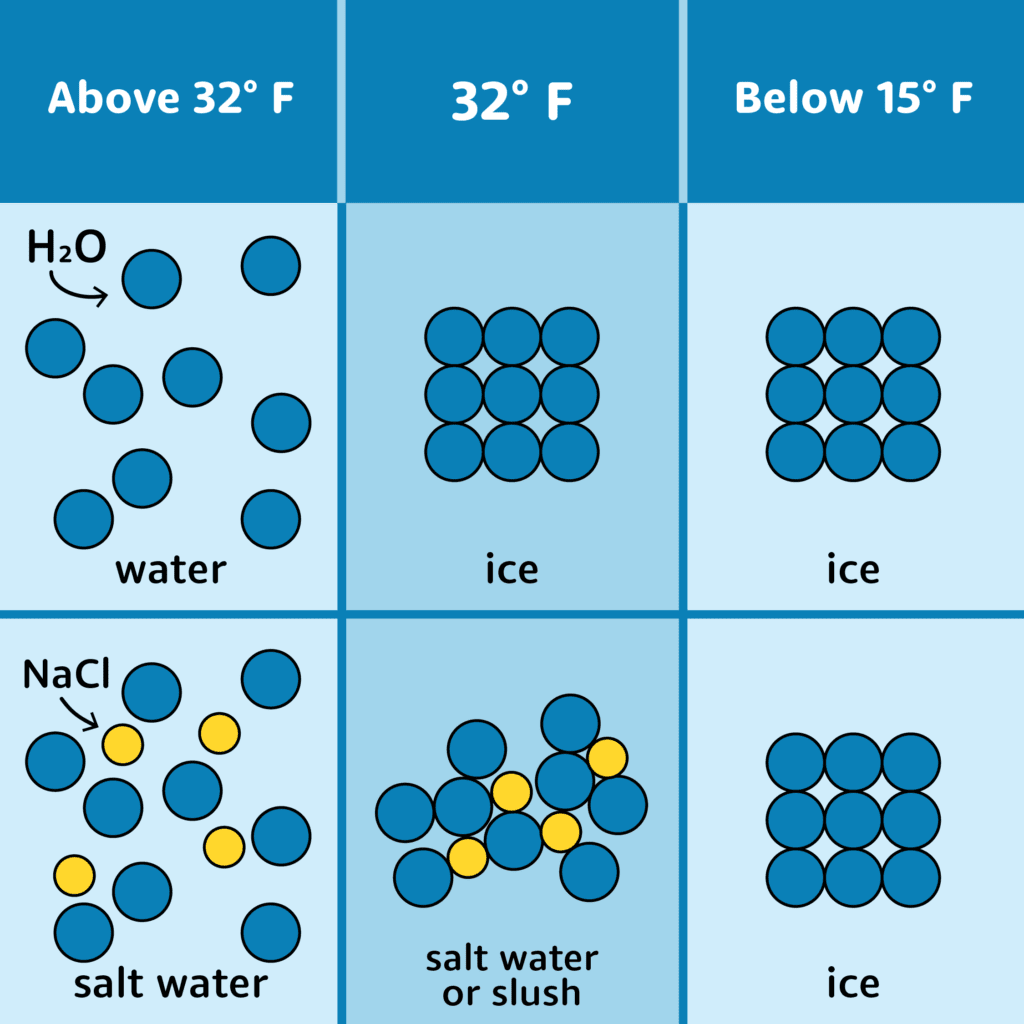
Why Do You Put Salt In Ice Water . As the op states, this lowers the freezing point of the liquid. In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. Why does ice water get colder when salt is added? This phenomenon is called freezing point depression. Why does the temperature get lower? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. The system is no longer at.
from dkijlydeeco.blob.core.windows.net
In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. This phenomenon is called freezing point depression. The system is no longer at. Why does the temperature get lower? The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. Why does ice water get colder when salt is added? As the op states, this lowers the freezing point of the liquid.
Why Does Salt And Ice Freeze Water at Michelle Fisher blog
Why Do You Put Salt In Ice Water Why does ice water get colder when salt is added? Why does ice water get colder when salt is added? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. This phenomenon is called freezing point depression. The system is no longer at. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. As the op states, this lowers the freezing point of the liquid. Why does the temperature get lower?
From www.branchor.com
Why Does Salt Melt Ice? Understanding the Science and Benefits of Using Why Do You Put Salt In Ice Water In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. The system is no longer at. This phenomenon is called freezing point depression. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has. Why Do You Put Salt In Ice Water.
From www.slideserve.com
PPT Why Do We Add Salt to Ice? PowerPoint Presentation, free download Why Do You Put Salt In Ice Water The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. In both cases, the answer is based on the fact that adding salt to an. Why Do You Put Salt In Ice Water.
From www.youtube.com
Why do you put salt in whitewash? YouTube Why Do You Put Salt In Ice Water As the op states, this lowers the freezing point of the liquid. The system is no longer at. In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. The. Why Do You Put Salt In Ice Water.
From doesitsnowin.com
Why Does Salt Melt Ice? Everything You Need To Know Why Do You Put Salt In Ice Water This phenomenon is called freezing point depression. Why does ice water get colder when salt is added? The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. As the op states, this lowers the freezing point of the liquid. Similar to. Why Do You Put Salt In Ice Water.
From lessonfullbeddington.z21.web.core.windows.net
Facts About Salt Melting Ice Why Do You Put Salt In Ice Water Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. Why does ice water get colder when salt is added? Why does the temperature get lower? In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. The system is. Why Do You Put Salt In Ice Water.
From musicbykatie.com
Does Salt Make Ice Melt The Fastest? Best 28 Answer Why Do You Put Salt In Ice Water As the op states, this lowers the freezing point of the liquid. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. Why does ice water get colder when salt is added? The system is no longer at. The actual reason that the application of salt causes ice to melt is that a solution. Why Do You Put Salt In Ice Water.
From sciencing.com
Experiments With Salt Melting Ice Sciencing Why Do You Put Salt In Ice Water In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. As the op states, this lowers the freezing point of the liquid. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a. Why Do You Put Salt In Ice Water.
From sciencing.com
What Happens When Salt Is Added to Water? Sciencing Why Do You Put Salt In Ice Water This phenomenon is called freezing point depression. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. Why does the temperature get lower? The system is no longer at. Why does ice water get colder when salt is added? As the op states, this lowers the freezing point of the liquid. In both cases,. Why Do You Put Salt In Ice Water.
From www.youtube.com
What happens when you put salt on ice cubes? YouTube Why Do You Put Salt In Ice Water Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. The system is no longer at. Why does the temperature get lower? In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. As the op states, this lowers the. Why Do You Put Salt In Ice Water.
From mirjamglessmer.com
Experiment Ice cubes melting in fresh water and salt water Why Do You Put Salt In Ice Water The system is no longer at. This phenomenon is called freezing point depression. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. As the op states, this lowers the freezing point of the liquid. Why does the temperature get lower? Why does ice water get colder when salt is added? In both cases,. Why Do You Put Salt In Ice Water.
From huntingwaterfalls.com
Why Does Salt Make Ice Colder? THE ACTUAL REASON Why Do You Put Salt In Ice Water This phenomenon is called freezing point depression. As the op states, this lowers the freezing point of the liquid. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. Why does ice water get colder when salt is added? The system is no longer at. In both cases, the answer is based on the. Why Do You Put Salt In Ice Water.
From lessonberginmoabites.z21.web.core.windows.net
How To Keep Ice From Melting Using Salt Why Do You Put Salt In Ice Water Why does ice water get colder when salt is added? Why does the temperature get lower? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. This phenomenon is called freezing point depression. As the op states, this lowers the freezing point of the liquid. The system is no longer at. The actual reason. Why Do You Put Salt In Ice Water.
From health.sunnybrook.ca
Why you shouldn't take the salt and ice challenge Why Do You Put Salt In Ice Water Why does the temperature get lower? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. In both cases, the answer is based on the. Why Do You Put Salt In Ice Water.
From thoughtfullysustainable.com
How Does Salt Affect Ice? A Simple Science Experiment Thoughtfully Why Do You Put Salt In Ice Water Why does the temperature get lower? The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. The system is no longer at. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. As the op. Why Do You Put Salt In Ice Water.
From studyzonepatristic.z13.web.core.windows.net
Why Does Adding Salt Make Ice Colder Why Do You Put Salt In Ice Water The system is no longer at. This phenomenon is called freezing point depression. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. The actual reason that the application. Why Do You Put Salt In Ice Water.
From huntingwaterfalls.com
Does Salt Make Ice Last Longer? No, But Also Yes Why Do You Put Salt In Ice Water The system is no longer at. This phenomenon is called freezing point depression. Why does the temperature get lower? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or.. Why Do You Put Salt In Ice Water.
From materialmcgheepearter.z21.web.core.windows.net
What Happens When Salt And Ice Mix Why Do You Put Salt In Ice Water As the op states, this lowers the freezing point of the liquid. In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. The actual reason that the application of. Why Do You Put Salt In Ice Water.
From www.thoughtco.com
Do Ice Cubes Melt Faster in Water or Air? Why Do You Put Salt In Ice Water In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. The system is no longer at. Why does the temperature get lower? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. Why does ice water get colder when. Why Do You Put Salt In Ice Water.
From sciencenotes.org
Why Salt Makes Ice Colder How Cold Ice Gets Why Do You Put Salt In Ice Water Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. Why does the temperature get lower? The system is no longer at. This phenomenon is. Why Do You Put Salt In Ice Water.
From studyzonepatristic.z13.web.core.windows.net
Why Does Adding Salt To Ice Make It Melt Why Do You Put Salt In Ice Water The system is no longer at. This phenomenon is called freezing point depression. Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. Why does the temperature get lower? In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or.. Why Do You Put Salt In Ice Water.
From www.youtube.com
The Science Behind Salt in Making Ice Cream YouTube Why Do You Put Salt In Ice Water In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. This phenomenon is called freezing point depression. As the op states, this lowers the freezing point of the liquid. Why does ice water get colder when salt is added? The system is no longer at.. Why Do You Put Salt In Ice Water.
From www.science-sparks.com
Chemistry for Kids Ice Experiments Science Sparks Why Do You Put Salt In Ice Water The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. As the op states, this lowers the freezing point of the liquid. This phenomenon is called freezing point depression. Why does the temperature get lower? The system is no longer at.. Why Do You Put Salt In Ice Water.
From sealevel.nasa.gov
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass Why Do You Put Salt In Ice Water In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. This phenomenon is called freezing point depression. As the op states, this lowers the freezing point of the liquid. Why does ice water get colder when salt is added? The system is no longer at.. Why Do You Put Salt In Ice Water.
From fox17.com
Behind the Science Why Salt is Used to Melt Ice WZTV Why Do You Put Salt In Ice Water The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. Why does the temperature get lower? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. The system is no longer at. In both cases,. Why Do You Put Salt In Ice Water.
From sciencenotes.org
Is Dissolving Salt in Water a Chemical Change or a Physical Change? Why Do You Put Salt In Ice Water The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. This phenomenon is called freezing point depression. Why does the temperature get lower? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. Why does. Why Do You Put Salt In Ice Water.
From www.studyladder.com
Experiment What effect does salt have on ice? Studyladder Why Do You Put Salt In Ice Water Why does the temperature get lower? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. This phenomenon is called freezing point depression. The system is no longer at. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing. Why Do You Put Salt In Ice Water.
From dkijlydeeco.blob.core.windows.net
Why Does Salt And Ice Freeze Water at Michelle Fisher blog Why Do You Put Salt In Ice Water The system is no longer at. Why does ice water get colder when salt is added? Why does the temperature get lower? The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. As the op states, this lowers the freezing point. Why Do You Put Salt In Ice Water.
From pagingfunmums.com
Melting Ice & Salt Science Experiment Why Do You Put Salt In Ice Water Why does ice water get colder when salt is added? Why does the temperature get lower? As the op states, this lowers the freezing point of the liquid. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. This phenomenon is. Why Do You Put Salt In Ice Water.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer Why Do You Put Salt In Ice Water The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. Why does ice water get colder. Why Do You Put Salt In Ice Water.
From way2estudy.blogspot.com
eduworld Why Does Salt Melt Ice? Why Do You Put Salt In Ice Water Why does the temperature get lower? Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. As the op states, this lowers the freezing point. Why Do You Put Salt In Ice Water.
From www.youtube.com
What happens when you put salt on ice YouTube Why Do You Put Salt In Ice Water Why does the temperature get lower? This phenomenon is called freezing point depression. In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. The system is no longer at. As the op states, this lowers the freezing point of the liquid. Why does ice water. Why Do You Put Salt In Ice Water.
From www.youtube.com
Science Sunday Melting ice in salt water YouTube Why Do You Put Salt In Ice Water Similar to sugar, salt affects how water freezes and effectively lowers the freezing/melting point of water. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. This phenomenon is called freezing point depression. In both cases, the answer is based on. Why Do You Put Salt In Ice Water.
From www.sciencekiddo.com
Why Salt Melts Ice Easy Science for Kids Science Kiddo Why Do You Put Salt In Ice Water Why does the temperature get lower? In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. Why does ice water get colder when salt is added? The actual reason that the application of salt causes ice to melt is that a solution of water and. Why Do You Put Salt In Ice Water.
From techiescientist.com
Why Does Salt Make Ice Colder? Techiescientist Why Do You Put Salt In Ice Water The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. In both cases, the answer is based on the fact that adding salt to an ice water mixture in equilibrium, lowers the freezing point (or. This phenomenon is called freezing point. Why Do You Put Salt In Ice Water.
From huntingwaterfalls.com
Why Does Salt Melt Ice Faster Than Sugar? SCIENCE EXPLAINED Why Do You Put Salt In Ice Water The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. Why does ice water get colder when salt is added? As the op states, this lowers the freezing point of the liquid. The system is no longer at. This phenomenon is. Why Do You Put Salt In Ice Water.