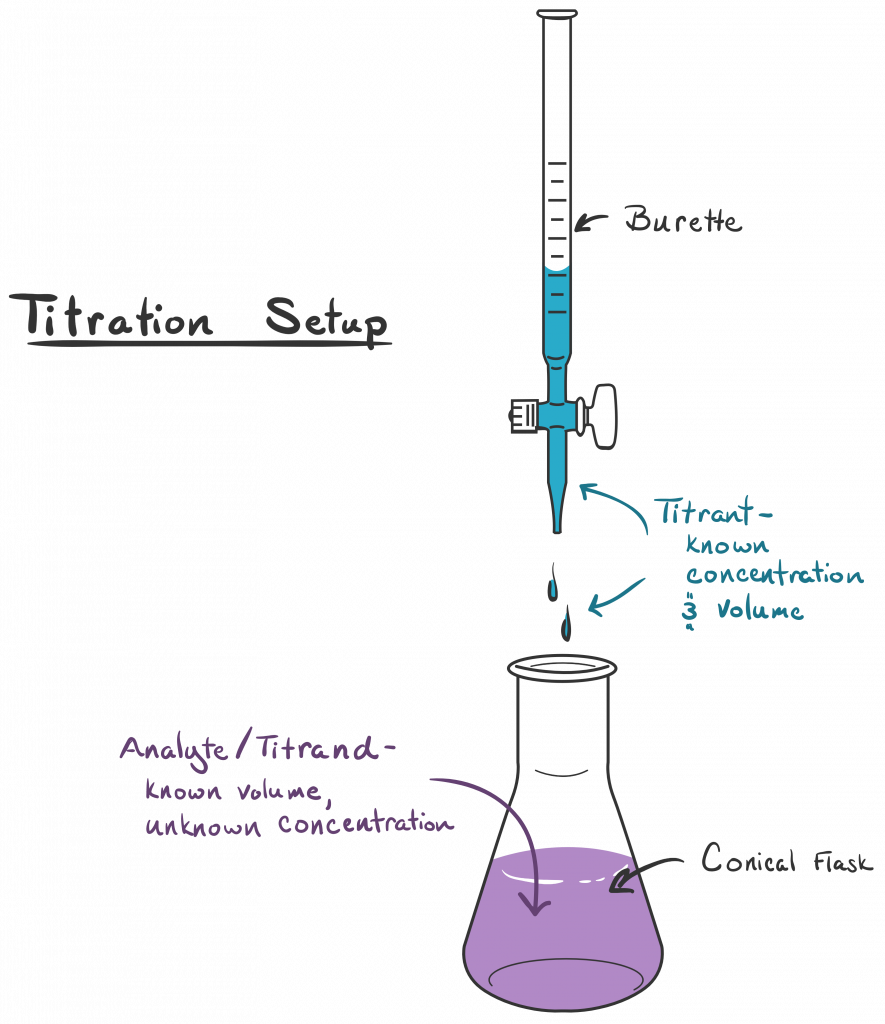
Titration Study Entresto . To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. Little is known about the dosing and. Access information about the three different dosing strengths and titration of entresto. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. Patients were then randomized to. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. See full prescribing & safety information, including.
from www.microlit.com
To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. Patients were then randomized to. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. Access information about the three different dosing strengths and titration of entresto. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. See full prescribing & safety information, including. Little is known about the dosing and. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition.
An Advanced Guide to Titration Microlit
Titration Study Entresto Little is known about the dosing and. See full prescribing & safety information, including. Little is known about the dosing and. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. Patients were then randomized to. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. Access information about the three different dosing strengths and titration of entresto.
From chem-textbook.ucalgary.ca
Titration Calculations UCalgary Chemistry Textbook Titration Study Entresto Access information about the three different dosing strengths and titration of entresto. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. The starting dose of sacubitril/valsartan is. Titration Study Entresto.
From vdocuments.mx
Titration Prelab. What is titration? Method to determine the Titration Study Entresto Little is known about the dosing and. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. Patients were then randomized to. Access information about the three different dosing strengths and titration of entresto. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. All patients with hfref were requested to. Titration Study Entresto.
From hendejac.github.io
Continuous ConstantpH Molecular Dynamics Analysis A Python Library Titration Study Entresto Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients. Titration Study Entresto.
From twitter.com
فدوى الخريصي on Twitter "Entresto initial dosing and titration 👇🏻 Titration Study Entresto Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. Patients were then randomized to. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. All patients with hfref were requested to receive. Titration Study Entresto.
From www.drugs.com
Entresto Package Insert Titration Study Entresto In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. See full prescribing & safety information, including. Patients were then randomized to. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. Access information about the three different. Titration Study Entresto.
From www.scribd.com
How To Prescribe ENTRESTO To Your Patients Dosing and Titration Guide Titration Study Entresto The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. See full prescribing & safety information, including. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. Access. Titration Study Entresto.
From www.researchgate.net
1 H NMR titration study of DFP into a buffered solution of H W •OH Titration Study Entresto See full prescribing & safety information, including. Access information about the three different dosing strengths and titration of entresto. Little is known about the dosing and. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition.. Titration Study Entresto.
From www.mometrix.com
Titration Chemistry Review (Video) Titration Study Entresto Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later.. Titration Study Entresto.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Study Entresto The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. Patients were then randomized to. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. Titration and tolerability of sacubitril/valsartan. Titration Study Entresto.
From studylib.net
entresto Titration Study Entresto Little is known about the dosing and. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. See full prescribing & safety information,. Titration Study Entresto.
From www.slideserve.com
PPT PAP Titration PowerPoint Presentation, free download ID2287369 Titration Study Entresto To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. See full prescribing & safety information, including. Patients were then randomized to. Little is known about the dosing and. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. Access information about the three different dosing strengths and titration. Titration Study Entresto.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Study Entresto Patients were then randomized to. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. Access information about the three different dosing strengths and titration of entresto. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. All patients with hfref were requested to receive follow‐up echocardiogram 12 months. Titration Study Entresto.
From testbook.com
Karl Fischer Titration Know its Definition, Example, Procedure Titration Study Entresto The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. See full prescribing & safety information, including. In our study involving patients with. Titration Study Entresto.
From www.researchgate.net
A titration study at physiological pH A titration study was conducted Titration Study Entresto To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. Patients were then randomized to. Access information about the three different dosing strengths and titration of entresto. Little is known about the dosing and. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every. Titration Study Entresto.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Study Entresto In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. See full prescribing & safety information, including. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2. Titration Study Entresto.
From www.dreamstime.com
Titration Technique in the Laboratory. Stock Image Image of analyzing Titration Study Entresto To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. See full prescribing & safety information, including. Patients were then randomized to. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. The starting dose of. Titration Study Entresto.
From mungfali.com
Titration Steps Titration Study Entresto Access information about the three different dosing strengths and titration of entresto. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily. Titration Study Entresto.
From www.jove.com
AcidBase Titration Curves JoVE Titration Study Entresto In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. Little is known about the dosing and. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. Access information about the three different dosing strengths and titration of entresto. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical. Titration Study Entresto.
From www.bestcpapcleaner.com
CPAP Titration Study (Meaning & Effects) Best CPAP Cleaner Titration Study Entresto The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. Little is known about the dosing and. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. To assess tolerability. Titration Study Entresto.
From chemistry-acidsandbases.weebly.com
Titrations Titration Study Entresto Little is known about the dosing and. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. In. Titration Study Entresto.
From docs.google.com
Titration practice.pdf Google Drive Titration Study Entresto To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. Titration and tolerability of sacubitril/valsartan for patients with. Titration Study Entresto.
From study.com
Quiz & Worksheet Overview of Titration Titration Study Entresto Patients were then randomized to. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. See full prescribing & safety information, including. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. Little is known about the dosing. Titration Study Entresto.
From www.vrogue.co
A Comparison Of Original Titration Curve And Calculat vrogue.co Titration Study Entresto Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. Patients were then randomized to. All patients with hfref were requested to receive. Titration Study Entresto.
From www.dreamstime.com
Titration Technique in the Laboratory. Stock Photo Image of fluid Titration Study Entresto Patients were then randomized to. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. Access information about the three different dosing strengths and titration of entresto. Little is known about the dosing and. To assess tolerability and optimal time point. Titration Study Entresto.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Study Entresto To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. Patients were then randomized to. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. See full prescribing & safety information, including. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose. Titration Study Entresto.
From www.cpap.com
What Is a CPAP Titration Study and Do You Need One? How Often? What Titration Study Entresto Little is known about the dosing and. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. Patients were then randomized to. All. Titration Study Entresto.
From www.researchgate.net
UVVis titration study of RhBC (3.5 × 105 M) with A Cu²⁺, (C) Hg²⁺ ion Titration Study Entresto Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. See full prescribing & safety information, including. Little is known about the dosing and. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose. Titration Study Entresto.
From www.researchgate.net
Renal Titration studies Maximal Transport for Glucose (TmG) and Renal Titration Study Entresto Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. Patients were then randomized to. Little is known about the dosing and. See full prescribing & safety information, including. To assess tolerability and optimal time point for initiation of sacubitril/valsartan in patients stabilised after acute heart failure. All patients with hfref were requested to receive follow‐up echocardiogram. Titration Study Entresto.
From www.researchgate.net
1 H NMR titration study. From bottom to top, 10 in CDCl 3 (1 mM Titration Study Entresto The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. Patients were then randomized to. Little is known about the dosing and. To. Titration Study Entresto.
From www.drugs.com
Entresto FDA prescribing information, side effects and uses Titration Study Entresto See full prescribing & safety information, including. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. Patients were then randomized to. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. Access information about the three different. Titration Study Entresto.
From www.researchgate.net
Left Titration study showing increase in fluorescence intensity with Titration Study Entresto Little is known about the dosing and. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily. Titration Study Entresto.
From app.jove.com
Complexometric EDTA Titration Curves Concept Analytical Chemistry Titration Study Entresto The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. Titration and tolerability of sacubitril/valsartan for patients with heart failure. Titration Study Entresto.
From www.sleepdrs.com
CPAP Titration Study · Pulmonary & Sleep Center of the Valley Titration Study Entresto In our study involving patients with chronic heart failure and a reduced ejection fraction, the inhibition. Access information about the three different dosing strengths and titration of entresto. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. See full prescribing & safety information, including. Little is known about the dosing and. All patients with hfref were. Titration Study Entresto.
From www.savemyexams.com
Prepare a Salt by Titration Edexcel GCSE Chemistry Revision Notes 2018 Titration Study Entresto See full prescribing & safety information, including. All patients with hfref were requested to receive follow‐up echocardiogram 12 months later. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. To assess tolerability and optimal time. Titration Study Entresto.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Study Entresto The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice. Patients were then randomized to. To assess tolerability and optimal time point for. Titration Study Entresto.