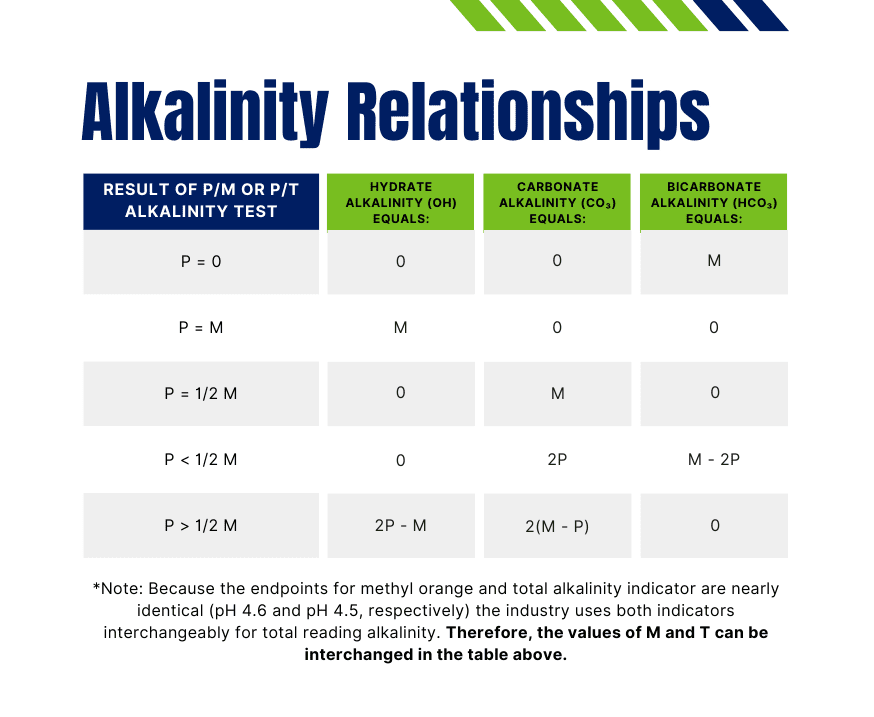
What Is P Alkalinity . Conversely, m alkalinity is less sensitive to such. Here are the key differences: Here, “m” refers to the ph indicator methylorange. Alkalinities are classified according to the endpoint of titration with strong acid: Alkalinity of water means acid neutralization capacity of water. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems.
from www.getchemready.com
P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Alkalinity of water means acid neutralization capacity of water. Conversely, m alkalinity is less sensitive to such. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Alkalinities are classified according to the endpoint of titration with strong acid: M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Here are the key differences: Here, “m” refers to the ph indicator methylorange.
Alkalinity in Boiler Water ChemREADY
What Is P Alkalinity M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Here, “m” refers to the ph indicator methylorange. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Conversely, m alkalinity is less sensitive to such. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Alkalinity of water means acid neutralization capacity of water. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Here are the key differences: Alkalinities are classified according to the endpoint of titration with strong acid:
From www.poolcalculator.com
Relationship Between pH and Total Alkalinity Pool Calculator What Is P Alkalinity Here, “m” refers to the ph indicator methylorange. Here are the key differences: Alkalinity of water means acid neutralization capacity of water. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Conversely, m alkalinity is less sensitive to such. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in. What Is P Alkalinity.
From www.youtube.com
Experiment Alkalinity of water PAlkalinityMAlkalinity water What Is P Alkalinity When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Conversely, m alkalinity is less sensitive to such. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems.. What Is P Alkalinity.
From www.pinterest.jp
Alkalinity PH chart with water acidity from bad to ideal outline What Is P Alkalinity Conversely, m alkalinity is less sensitive to such. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Alkalinities are classified according to the endpoint of titration with strong acid: Here are the key differences: Alkalinity of water means acid neutralization capacity of water. When you add acid in water (adding. What Is P Alkalinity.
From dynamixinc.com
PH Alkalinity Mixing With Reaction Times Dynamix Agitators What Is P Alkalinity When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Conversely, m alkalinity is less sensitive to such.. What Is P Alkalinity.
From www.whoi.edu
Ocean Alkalinity Woods Hole Oceanographic Institution What Is P Alkalinity P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Here are the key differences: When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Alkalinities are classified according to the endpoint of titration with strong acid: Conversely, m alkalinity is less sensitive to such. Here, “m”. What Is P Alkalinity.
From distinctionbetween.com
What is Difference Between p Alkalinity and m Alkalinity What Is P Alkalinity P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Alkalinities are classified according to the endpoint of titration with strong acid: When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Conversely, m alkalinity is less sensitive to such.. What Is P Alkalinity.
From www.slideserve.com
PPT Alkalinity PowerPoint Presentation, free download ID9437546 What Is P Alkalinity Here are the key differences: P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Conversely, m alkalinity is less sensitive to such. M alkalinity and p alkalinity are both. What Is P Alkalinity.
From www.slideserve.com
PPT What is Alkalinity? PowerPoint Presentation, free download ID What Is P Alkalinity P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. Alkalinities are classified according to the endpoint. What Is P Alkalinity.
From slideplayer.com
Lecture Goals To review how pH and alkalinity work. ppt video online What Is P Alkalinity P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Here, “m” refers to the ph indicator methylorange. Alkalinity of water means acid neutralization capacity of water. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Alkalinities are classified according to the endpoint. What Is P Alkalinity.
From www.youtube.com
P Alkalinity and M Alkalinity in water YouTube What Is P Alkalinity Here are the key differences: P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Here, “m” refers to the ph indicator methylorange. Alkalinity of water means acid neutralization capacity of water. Mainly, it is due to. What Is P Alkalinity.
From differencebetweenz.com
Difference between Alkalinity and pH Difference Betweenz What Is P Alkalinity Alkalinities are classified according to the endpoint of titration with strong acid: P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. Alkalinity of water means acid neutralization capacity. What Is P Alkalinity.
From www.slideserve.com
PPT Alkalinity PowerPoint Presentation, free download ID4752704 What Is P Alkalinity Here, “m” refers to the ph indicator methylorange. Here are the key differences: When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Conversely, m. What Is P Alkalinity.
From www.youtube.com
Trick for Alkalinity in water viva questions IIT JEE , B.Tech and B What Is P Alkalinity Here, “m” refers to the ph indicator methylorange. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. Conversely, m alkalinity is less sensitive to such. P alkalinity is sensitive to ph changes and can provide. What Is P Alkalinity.
From blog.orendatech.com
What is Alkalinity? What Is P Alkalinity Here, “m” refers to the ph indicator methylorange. Here are the key differences: When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Alkalinities are. What Is P Alkalinity.
From pediaa.com
What is the Difference Between PAlkalinity and MAlkalinity What Is P Alkalinity Conversely, m alkalinity is less sensitive to such. Here, “m” refers to the ph indicator methylorange. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Here are the key differences: When you add acid in water (adding h. What Is P Alkalinity.
From www.slidemake.com
Alkalinity Of Water Experiment Presentation What Is P Alkalinity Conversely, m alkalinity is less sensitive to such. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Alkalinities are classified according to the endpoint of titration with strong acid: Here, “m” refers to the ph indicator methylorange. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Mainly,. What Is P Alkalinity.
From www.slideserve.com
PPT Alkalinity PowerPoint Presentation, free download ID607384 What Is P Alkalinity Here are the key differences: P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Here, “m” refers to the ph indicator methylorange. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Alkalinity of water means. What Is P Alkalinity.
From www.worksheetsplanet.com
What is Alkalinity Definition of Alkalinity What Is P Alkalinity Here are the key differences: P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Conversely, m alkalinity is less sensitive to such. Alkalinity of water means acid neutralization capacity of water. Here, “m” refers to the ph indicator methylorange. When you add acid in water (adding h + ions) water. What Is P Alkalinity.
From www.youtube.com
How to determine alkalinity of water? Experiment on Alkalinity of What Is P Alkalinity Here are the key differences: M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Here, “m” refers to the ph indicator methylorange. Conversely, m alkalinity is. What Is P Alkalinity.
From gamesmartz.com
Alkalinity Definition & Image GameSmartz What Is P Alkalinity Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. Conversely, m alkalinity is less sensitive to such. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. M alkalinity and p alkalinity are both measures of. What Is P Alkalinity.
From www.slideserve.com
PPT Alkalinity PowerPoint Presentation, free download ID607384 What Is P Alkalinity Conversely, m alkalinity is less sensitive to such. Here, “m” refers to the ph indicator methylorange. Alkalinities are classified according to the endpoint of titration with strong acid: Here are the key differences: M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. P alkalinity is. What Is P Alkalinity.
From www.slideserve.com
PPT Alkalinity and hardness PowerPoint Presentation, free download What Is P Alkalinity Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. Conversely, m alkalinity is less sensitive to such. Alkalinities are classified according to the endpoint of titration with strong acid: M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in. What Is P Alkalinity.
From www.slideserve.com
PPT Alkalinity of water PowerPoint Presentation, free download ID What Is P Alkalinity Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. Here, “m” refers to the ph indicator methylorange. Alkalinity of water means acid neutralization capacity of water. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. When you add acid in water (adding h +. What Is P Alkalinity.
From www.slideserve.com
PPT WHAT IS ALKALINITY OF WATER? PowerPoint Presentation, free What Is P Alkalinity When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Here are the key differences: M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. P alkalinity is the alkalinity that is determined by using. What Is P Alkalinity.
From www.membranechemicals.com
Understanding Alkalinity and its Impact on Reverse Osmosis Membranes What Is P Alkalinity P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Here are the key differences: Alkalinity of water means acid neutralization capacity of water. Mainly, it is due to carbonate, bicarbonate & hydroxide ion. What Is P Alkalinity.
From www.youtube.com
Calculation of Alkalinity of Water Part 2 to determine alkalinity of What Is P Alkalinity Here are the key differences: Alkalinities are classified according to the endpoint of titration with strong acid: Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions present in water. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph.. What Is P Alkalinity.
From www.getchemready.com
Alkalinity in Boiler Water ChemREADY What Is P Alkalinity Conversely, m alkalinity is less sensitive to such. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms. What Is P Alkalinity.
From www.thewastewaterblog.com
pH & Alkalinity What Is P Alkalinity Alkalinities are classified according to the endpoint of titration with strong acid: Alkalinity of water means acid neutralization capacity of water. Conversely, m alkalinity is less sensitive to such. Here, “m” refers to the ph indicator methylorange. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Here are. What Is P Alkalinity.
From pdfprof.com
alkalinity measurement What Is P Alkalinity Conversely, m alkalinity is less sensitive to such. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. Here are the key differences: Alkalinity of water means acid neutralization capacity of water. Here, “m” refers to the ph indicator. What Is P Alkalinity.
From distinctionbetween.com
What is Difference Between p Alkalinity and m Alkalinity What Is P Alkalinity Alkalinity of water means acid neutralization capacity of water. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Conversely, m alkalinity is less sensitive to such. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or the mixture of two ions. What Is P Alkalinity.
From www.slideserve.com
PPT Alkalinity and hardness PowerPoint Presentation, free download What Is P Alkalinity M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. Alkalinity of water means acid neutralization capacity of water. Here are the key differences: P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Alkalinities are classified according to the endpoint of titration with. What Is P Alkalinity.
From www.slideserve.com
PPT Alkalinity PowerPoint Presentation, free download ID607384 What Is P Alkalinity When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Alkalinity of water means acid neutralization capacity of water. P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water. What Is P Alkalinity.
From blog.orendatech.com
What is Alkalinity? What Is P Alkalinity Here are the key differences: P alkalinity is sensitive to ph changes and can provide insights into the carbon dioxide balance in water systems. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. P alkalinity is the alkalinity that is determined by using phenolphthalein indicator.. What Is P Alkalinity.
From www.difference.wiki
p Alkalinity vs. m Alkalinity What’s the Difference? What Is P Alkalinity Alkalinity of water means acid neutralization capacity of water. M alkalinity and p alkalinity are both measures of the buffering capacity of water, but they differ in terms of the specific components they. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Here, “m” refers to the ph. What Is P Alkalinity.
From netsolwater.com
What is the relationship between pH and alkalinity What Is P Alkalinity Here, “m” refers to the ph indicator methylorange. When you add acid in water (adding h + ions) water absorbs h + ions without showing significant change in ph. Here are the key differences: P alkalinity is the alkalinity that is determined by using phenolphthalein indicator. Mainly, it is due to carbonate, bicarbonate & hydroxide ion present in water or. What Is P Alkalinity.