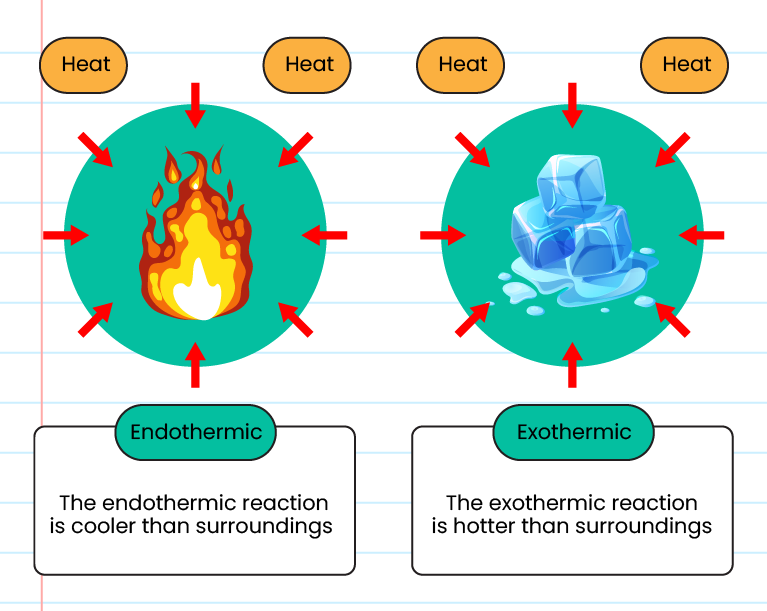
Endothermic Reaction And Temperature Change . endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). endothermic reactions result in an overall positive heat of reaction (qrxn> 0). These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Changes in heat energy accompany salts dissolving in water. an endothermic reaction or physical change is one in which the system takes in heat from the environment rather than. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. use this experiment and accompanying student worksheet to boost your students’ understanding of chemical energetics and. an increase in temperature favours the endothermic reaction. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. revision notes on required practical: endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Other chemical reactions release energy. the heat of reaction is the change in the enthalpy of a chemical reaction. when energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which. when energy is released in an exothermic reaction, the temperature of the reaction mixture increases.
from diagramdataconfusion.z22.web.core.windows.net
When energy is absorbed in an endothermic reaction, the. exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for. introduce students to chemical energetics by measuring temperature changes in a range of chemical reactions with a class practical such as ‘exothermic or endothermic?’. exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When temperature is the stress that affects a system at. the video explains that at the end of the reaction you should start to see the temperature level off or decrease. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. explain how temperature changes affect a system at equilibrium. In the above equilibrium, the enthalpy change shows. an endothermic reaction or physical change is one in which the system takes in heat from the environment rather than.
Endothermic Energy Diagram
Endothermic Reaction And Temperature Change endothermic reactions result in an overall positive heat of reaction (qrxn> 0). Changes in heat energy accompany salts dissolving in water. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. an increase in temperature favours the endothermic reaction. revision notes on required practical: in exothermic reactions, heat is released, whereas heat is absorbed in endothermic reactions. the heat of reaction is the change in the enthalpy of a chemical reaction. When energy is absorbed in an endothermic reaction, the. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. introduce students to chemical energetics by measuring temperature changes in a range of chemical reactions with a class practical such as ‘exothermic or endothermic?’. exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases.
From www.numerade.com
SOLVED Which one of the following given graphs represents the Endothermic Reaction And Temperature Change an increase in temperature favours the endothermic reaction. when energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. When energy is absorbed in an endothermic reaction, the. Changes in heat energy accompany. Investigating temperature changes for. Endothermic Reaction And Temperature Change.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction And Temperature Change Investigating temperature changes for the aqa gcse chemistry syllabus, written by. use this experiment and accompanying student worksheet to boost your students’ understanding of chemical energetics and. When temperature is the stress that affects a system at. These are reactions that take in energy from the surroundings (ie energy en ters the reaction, which will help. introduce students. Endothermic Reaction And Temperature Change.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction And Temperature Change Investigating temperature changes for the aqa gcse chemistry syllabus, written by. when energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Changes in heat energy accompany salts dissolving in water. Changes in heat energy accompany. use this experiment and accompanying student worksheet to boost your students’ understanding of chemical energetics and. These reactions. Endothermic Reaction And Temperature Change.
From schematiclibsalamis77.z13.web.core.windows.net
Enthalpy Diagram For Endothermic Reaction Endothermic Reaction And Temperature Change explain how temperature changes affect a system at equilibrium. revision notes on required practical: in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. when energy is taken in from the surroundings, this is. Endothermic Reaction And Temperature Change.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Reaction And Temperature Change endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). the heat of reaction is the change in the enthalpy of a chemical reaction. use this experiment and accompanying student worksheet to boost your students’ understanding of chemical energetics and. endothermic reactions are chemical reactions in which the reactants. Endothermic Reaction And Temperature Change.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic Reaction Energy Profile Diagram Endothermic Reaction And Temperature Change when energy is released in an exothermic reaction, the temperature of the reaction mixture increases. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. introduce students to chemical energetics by measuring temperature changes in a range of chemical reactions with a class practical such as. Endothermic Reaction And Temperature Change.
From www.vrogue.co
Endothermic Reactions The Science Behind Temperature vrogue.co Endothermic Reaction And Temperature Change endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. when energy is released in an exothermic reaction, the temperature of the reaction mixture increases. endothermic reactions result in an overall positive heat of reaction (qrxn> 0). introduce students to chemical energetics by measuring temperature. Endothermic Reaction And Temperature Change.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction And Temperature Change the heat of reaction is the change in the enthalpy of a chemical reaction. exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for. Changes in heat energy accompany salts dissolving in water. introduce students to chemical energetics by measuring temperature changes in a range of chemical reactions with. Endothermic Reaction And Temperature Change.
From lessonschoolallodial.z13.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction And Temperature Change endothermic reactions result in an overall positive heat of reaction (qrxn> 0). in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Changes in heat energy accompany salts dissolving in water. When. Endothermic Reaction And Temperature Change.
From gizmo.ai
Chemistry Unit 3 Fertilisers Flashcards Endothermic Reaction And Temperature Change in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. use this experiment and accompanying student worksheet to boost your students’ understanding of chemical energetics and. endothermic reactions. Endothermic Reaction And Temperature Change.
From hxeuornzy.blob.core.windows.net
How Does Pressure Affect Phase Change at Benjamin Goodwin blog Endothermic Reaction And Temperature Change when energy is released in an exothermic reaction, the temperature of the reaction mixture increases. In the above equilibrium, the enthalpy change shows. Changes in heat energy accompany. in exothermic reactions, heat is released, whereas heat is absorbed in endothermic reactions. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the. Endothermic Reaction And Temperature Change.
From diagramdataconfusion.z22.web.core.windows.net
Endothermic Energy Diagram Endothermic Reaction And Temperature Change Changes in heat energy accompany. Changes in heat energy accompany salts dissolving in water. These are reactions that take in energy from the surroundings (ie energy en ters the reaction, which will help. introduce students to chemical energetics by measuring temperature changes in a range of chemical reactions with a class practical such as ‘exothermic or endothermic?’. endothermic. Endothermic Reaction And Temperature Change.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Endothermic Reaction And Temperature Change endothermic reactions result in an overall positive heat of reaction (qrxn> 0). use this experiment and accompanying student worksheet to boost your students’ understanding of chemical energetics and. revision notes on required practical: an endothermic reaction or physical change is one in which the system takes in heat from the environment rather than. Changes in heat. Endothermic Reaction And Temperature Change.
From gizmo.ai
5 Energy Changes Flashcards Endothermic Reaction And Temperature Change These are reactions that take in energy from the surroundings (ie energy en ters the reaction, which will help. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Changes in heat energy accompany. the heat of reaction is the change in the enthalpy of a chemical reaction. use this. Endothermic Reaction And Temperature Change.
From www.answersarena.com
[Solved] At what temp A reaction is endothermic and spont Endothermic Reaction And Temperature Change When temperature is the stress that affects a system at. when energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When energy is absorbed in an endothermic reaction, the. introduce students to chemical energetics by measuring temperature changes in a range of chemical reactions with a class practical such as ‘exothermic or endothermic?’.. Endothermic Reaction And Temperature Change.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction And Temperature Change an endothermic reaction or physical change is one in which the system takes in heat from the environment rather than. explain how temperature changes affect a system at equilibrium. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. These are reactions that take in energy. Endothermic Reaction And Temperature Change.
From circuitwiringtray.z13.web.core.windows.net
Exothermic And Endothermic Energy Diagrams Endothermic Reaction And Temperature Change When temperature is the stress that affects a system at. exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. When energy is absorbed in an endothermic reaction, the. Changes in heat energy accompany salts dissolving in water. in exothermic reactions, heat is released, whereas heat is absorbed in endothermic reactions. exothermic reactions. Endothermic Reaction And Temperature Change.
From asenoveo6schematic.z14.web.core.windows.net
How To Read Energy Diagrams Chemistry Endothermic Reaction And Temperature Change endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. the video explains that at the end of the reaction you should start to see the temperature level off or decrease. explain how temperature changes affect a system at equilibrium. Changes in heat energy accompany salts dissolving in water.. Endothermic Reaction And Temperature Change.
From 2k28pjwirelib.z13.web.core.windows.net
Energy Diagram For Chemical Reaction Endothermic Reaction And Temperature Change endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. when energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. endothermic reactions are. Endothermic Reaction And Temperature Change.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction And Temperature Change Changes in heat energy accompany. use this experiment and accompanying student worksheet to boost your students’ understanding of chemical energetics and. in exothermic reactions, heat is released, whereas heat is absorbed in endothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). explain how temperature changes affect. Endothermic Reaction And Temperature Change.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction And Temperature Change These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). explain how temperature changes affect a system at equilibrium. When temperature is. Endothermic Reaction And Temperature Change.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction And Temperature Change In the above equilibrium, the enthalpy change shows. when energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which. exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. when energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When temperature. Endothermic Reaction And Temperature Change.
From sciencenotes.org
Exothermic Reactions Definition and Examples Endothermic Reaction And Temperature Change the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a. Changes in heat energy accompany salts dissolving in water. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. These are reactions that take in energy from the surroundings (ie energy. Endothermic Reaction And Temperature Change.
From edu.rsc.org
Temperature changes in exothermic and endothermic reactions Resource Endothermic Reaction And Temperature Change an endothermic reaction or physical change is one in which the system takes in heat from the environment rather than. use this experiment and accompanying student worksheet to boost your students’ understanding of chemical energetics and. an increase in temperature favours the endothermic reaction. These reactions lower the temperature of their surrounding area, thereby creating a cooling. Endothermic Reaction And Temperature Change.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction And Temperature Change When energy is absorbed in an endothermic reaction, the. Other chemical reactions release energy. When temperature is the stress that affects a system at. an increase in temperature favours the endothermic reaction. in exothermic reactions, heat is released, whereas heat is absorbed in endothermic reactions. endothermic and exothermic reactions can be thought of as having energy as. Endothermic Reaction And Temperature Change.
From mungfali.com
Exothermic Reaction Energy Profile Diagram Endothermic Reaction And Temperature Change an endothermic reaction or physical change is one in which the system takes in heat from the environment rather than. exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for. the rate of the endothermic reaction is increased more than the rate of the backward reaction in response to. Endothermic Reaction And Temperature Change.
From katiekruwmullins.blogspot.com
Explain Three Differences Between Exothermic and Endothermic Reactions Endothermic Reaction And Temperature Change in exothermic reactions, heat is released, whereas heat is absorbed in endothermic reactions. endothermic reactions result in an overall positive heat of reaction (qrxn> 0). the video explains that at the end of the reaction you should start to see the temperature level off or decrease. endothermic and exothermic reactions can be thought of as having. Endothermic Reaction And Temperature Change.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Endothermic Reaction And Temperature Change In the above equilibrium, the enthalpy change shows. exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for. These are reactions that take in energy from the surroundings (ie energy en ters the reaction, which will help. endothermic reactions are chemical reactions in which the reactants absorb heat energy from. Endothermic Reaction And Temperature Change.
From hxevvbgky.blob.core.windows.net
Endothermic Examples In Chemistry at Beverly Spain blog Endothermic Reaction And Temperature Change an increase in temperature favours the endothermic reaction. revision notes on required practical: In the above equilibrium, the enthalpy change shows. the video explains that at the end of the reaction you should start to see the temperature level off or decrease. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. . Endothermic Reaction And Temperature Change.
From wiredataremetev5.z4.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction And Temperature Change Other chemical reactions release energy. revision notes on required practical: These are reactions that take in energy from the surroundings (ie energy en ters the reaction, which will help. introduce students to chemical energetics by measuring temperature changes in a range of chemical reactions with a class practical such as ‘exothermic or endothermic?’. the heat of reaction. Endothermic Reaction And Temperature Change.
From www.chegg.com
Solved Question 18CO2+H2⇌CO+H2OIncreasing the temperature of Endothermic Reaction And Temperature Change the video explains that at the end of the reaction you should start to see the temperature level off or decrease. an endothermic reaction or physical change is one in which the system takes in heat from the environment rather than. revision notes on required practical: When temperature is the stress that affects a system at. Other. Endothermic Reaction And Temperature Change.
From www.chegg.com
Solved The change in what quantity determines whether a Endothermic Reaction And Temperature Change When energy is absorbed in an endothermic reaction, the. These are reactions that take in energy from the surroundings (ie energy en ters the reaction, which will help. exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. use this experiment and accompanying student worksheet to boost your students’ understanding of chemical energetics and.. Endothermic Reaction And Temperature Change.
From gizmo.ai
5 Energy Changes Flashcards Endothermic Reaction And Temperature Change These are reactions that take in energy from the surroundings (ie energy en ters the reaction, which will help. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. the video explains that at the. Endothermic Reaction And Temperature Change.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction And Temperature Change exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. when energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When temperature is the stress that affects a system at. introduce students to chemical energetics by measuring temperature changes in a range of chemical reactions with a class. Endothermic Reaction And Temperature Change.
From www.chegg.com
Solved Question 9A reaction that occurs at 80°C has a Endothermic Reaction And Temperature Change explain how temperature changes affect a system at equilibrium. When energy is absorbed in an endothermic reaction, the. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions result in an overall positive heat of reaction (qrxn> 0). the video explains that at the end of the reaction you should start. Endothermic Reaction And Temperature Change.