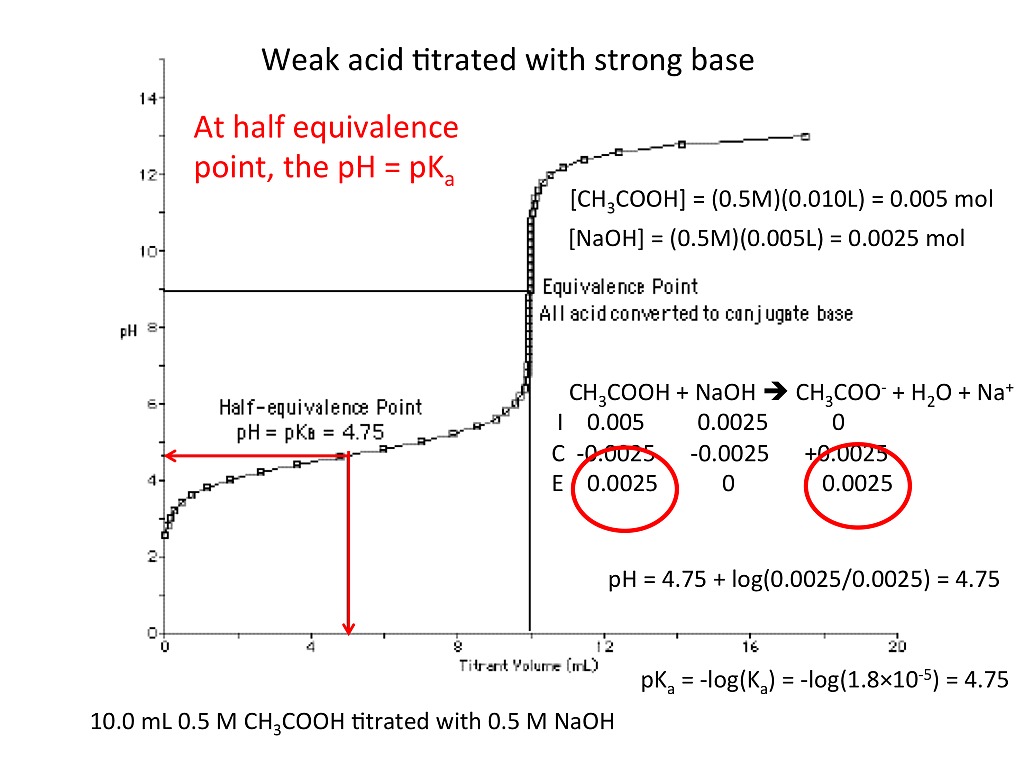
In A Titration Experiment Based On The Equation Above . The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar.
from mavink.com
5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
Titration Labeled
In A Titration Experiment Based On The Equation Above In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS In A Titration Experiment Based On The Equation Above In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The shape of a titration curve, a plot of ph versus the amount of acid or base added,. In A Titration Experiment Based On The Equation Above.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps In A Titration Experiment Based On The Equation Above The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. A titration is a volumetric technique in which. In A Titration Experiment Based On The Equation Above.
From bramblechemistry.weebly.com
4C6 Titration In A Titration Experiment Based On The Equation Above The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a tritration experiment based on the equation above, 25.0milliliters. In A Titration Experiment Based On The Equation Above.
From ar.inspiredpencil.com
Titration Setup Diagram In A Titration Experiment Based On The Equation Above In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. 5 fe2+ + mno4− + 8 h+ ⇄. In A Titration Experiment Based On The Equation Above.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps In A Titration Experiment Based On The Equation Above In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. A titration is a volumetric technique in which a solution. In A Titration Experiment Based On The Equation Above.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration In A Titration Experiment Based On The Equation Above A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a. In A Titration Experiment Based On The Equation Above.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation In A Titration Experiment Based On The Equation Above The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. 5 fe2+ + mno4− + 8 h+ ⇄ 5. In A Titration Experiment Based On The Equation Above.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. The above equation works only for neutralizations in which there. In A Titration Experiment Based On The Equation Above.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry In A Titration Experiment Based On The Equation Above In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The shape of a titration curve, a. In A Titration Experiment Based On The Equation Above.
From www.youtube.com
️ Titration Experiment for Board Class Complete Video to Understand Chemistry Practical In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a tritration experiment based on the equation above, 25.0milliliters of an. In A Titration Experiment Based On The Equation Above.
From solvedlib.com
A titration experiment was carried out to determine t… SolvedLib In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one. In A Titration Experiment Based On The Equation Above.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube In A Titration Experiment Based On The Equation Above A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 5 fe2+ + mno4− + 8 h+ ⇄ 5. In A Titration Experiment Based On The Equation Above.
From www.chegg.com
Solved QUESTION 1 A grade 12 learner performed a titration In A Titration Experiment Based On The Equation Above The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+. In A Titration Experiment Based On The Equation Above.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple In A Titration Experiment Based On The Equation Above A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. The above equation works only for neutralizations. In A Titration Experiment Based On The Equation Above.
From www.chegg.com
Solved Question 5 a) The results of a titration experiment In A Titration Experiment Based On The Equation Above The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a tritration experiment based on the equation above, 25.0milliliters. In A Titration Experiment Based On The Equation Above.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation ID6592809 In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. A titration. In A Titration Experiment Based On The Equation Above.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Foundation In A Titration Experiment Based On The Equation Above The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The shape of a titration curve, a plot of ph versus the. In A Titration Experiment Based On The Equation Above.
From theedge.com.hk
Chemistry How To Titration The Edge In A Titration Experiment Based On The Equation Above The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. A titration. In A Titration Experiment Based On The Equation Above.
From solvedlib.com
Colorimetric Titration Experiment Determine the dmoum… SolvedLib In A Titration Experiment Based On The Equation Above The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration. In A Titration Experiment Based On The Equation Above.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Education In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a tritration experiment based on the equation above, 25.0milliliters of an. In A Titration Experiment Based On The Equation Above.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Foundation In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a tritration experiment based on the equation above, 25.0milliliters of an. In A Titration Experiment Based On The Equation Above.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources In A Titration Experiment Based On The Equation Above The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. The above equation works only for neutralizations in. In A Titration Experiment Based On The Equation Above.
From mavink.com
Titration Labeled In A Titration Experiment Based On The Equation Above A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a tritration experiment based on the equation above,. In A Titration Experiment Based On The Equation Above.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts In A Titration Experiment Based On The Equation Above The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. The shape of a titration curve, a plot of ph versus the amount of acid or base added,. In A Titration Experiment Based On The Equation Above.
From www.youtube.com
Titration Experiment & Calculate the Molarity of Acetic Acid in Vinegar YouTube In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. The above. In A Titration Experiment Based On The Equation Above.
From studylib.net
Titration Experiment In A Titration Experiment Based On The Equation Above The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+. In A Titration Experiment Based On The Equation Above.
From www.chegg.com
Solved In a titration experiment (see image below), a 2 M In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. A titration is a volumetric technique in which a solution. In A Titration Experiment Based On The Equation Above.
From www.youtube.com
28 TITRATION 1 Experiment , Calculation HSC Chemistry YouTube In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The shape of a titration curve, a plot of ph versus the. In A Titration Experiment Based On The Equation Above.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent In A Titration Experiment Based On The Equation Above The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. In A Titration Experiment Based On The Equation Above.
From mungfali.com
Titration Lab Diagram In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. A titration is a volumetric technique in which a solution. In A Titration Experiment Based On The Equation Above.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d) TRIPLE Teaching Resources In A Titration Experiment Based On The Equation Above The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The shape of a titration curve, a plot of. In A Titration Experiment Based On The Equation Above.
From www.vecteezy.com
Acid base titration experiment and phases of color change during titration 20240684 Vector Art In A Titration Experiment Based On The Equation Above A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a. In A Titration Experiment Based On The Equation Above.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is occurring in solution during a titration. The above. In A Titration Experiment Based On The Equation Above.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali GCSE Chemistry Revision In A Titration Experiment Based On The Equation Above In a tritration experiment based on the equation above, 25.0milliliters of an acidified fe 2+ solution requires 14milliliters of standard o.o50 molar. 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The above equation works only for neutralizations in which there. In A Titration Experiment Based On The Equation Above.
From ar.inspiredpencil.com
Titration Setup Diagram In A Titration Experiment Based On The Equation Above 5 fe2+ + mno4− + 8 h+ ⇄ 5 fe3+ + mn2+ + 4 h2o in a titration experiment based on the equation above, 25.0 milliliters of an. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a volumetric technique in which a solution of one. In A Titration Experiment Based On The Equation Above.