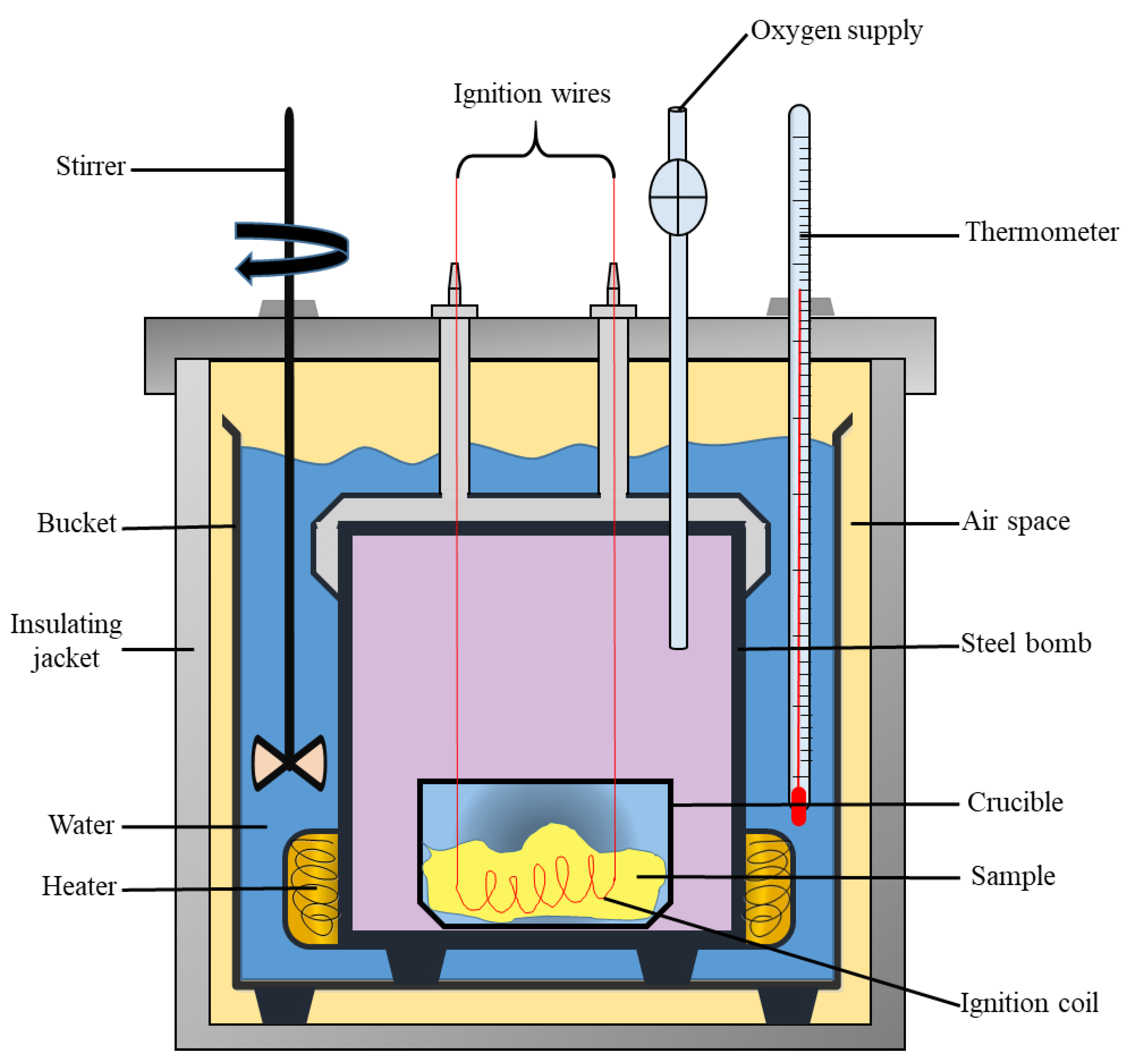
Calorimetry Enthalpy Of Reaction . In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. First, you need to be a little careful about whether the experiment was done at constant pressure or constant. A calorimeter can be made up of a polystyrene drinking cup, a. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. It uses devices called calorimeters,. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. Calculating the enthalpy change of reaction, hrfrom experimental data. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Measurement of an enthalpy change.
from www.animalia-life.club
The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. Measurement of an enthalpy change. Calculating the enthalpy change of reaction, hrfrom experimental data. First, you need to be a little careful about whether the experiment was done at constant pressure or constant. A calorimeter can be made up of a polystyrene drinking cup, a. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It uses devices called calorimeters,. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions.
Calorimeter Diagram
Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. Measurement of an enthalpy change. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. A calorimeter can be made up of a polystyrene drinking cup, a. First, you need to be a little careful about whether the experiment was done at constant pressure or constant. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calculating the enthalpy change of reaction, hrfrom experimental data. It uses devices called calorimeters,. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can.
From hxemseydn.blob.core.windows.net
How To Calculate Heat Of Calorimeter at Lucille Monahan blog Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. Measurement of an enthalpy change. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry. Calorimetry Enthalpy Of Reaction.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. Calculating the enthalpy change of reaction, hrfrom experimental data. Measurement of an enthalpy change. First, you need to be a little careful about whether. Calorimetry Enthalpy Of Reaction.
From www.animalia-life.club
Calorimeter Diagram Calorimetry Enthalpy Of Reaction To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for. Calorimetry Enthalpy Of Reaction.
From www.studocu.com
Calorimetry Prac CHEMISTRY CHEMISTRY UNIT 2 Investigation of Calorimetry Enthalpy Of Reaction First, you need to be a little careful about whether the experiment was done at constant pressure or constant. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calculating the enthalpy change of reaction, hrfrom experimental data. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy. Calorimetry Enthalpy Of Reaction.
From fyocdslfs.blob.core.windows.net
Calorimetry Explained at Edith Wallis blog Calorimetry Enthalpy Of Reaction It uses devices called calorimeters,. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. Calorimetry is a technique used to measure changes in enthalpy of. Calorimetry Enthalpy Of Reaction.
From www.chegg.com
Solved This question has now been updated,please Solve the Calorimetry Enthalpy Of Reaction Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. Calculating the enthalpy change of reaction, hrfrom experimental data. To determine the enthalpy, stability, heat capacity, and other thermochemical. Calorimetry Enthalpy Of Reaction.
From studylib.net
Enthalpy of Reaction and Calorimetry worksheet SCH4U1CCVI Calorimetry Enthalpy Of Reaction Calculating the enthalpy change of reaction, hrfrom experimental data. Measurement of an enthalpy change. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. A calorimeter can be made up of a polystyrene drinking cup, a. In this explainer, we. Calorimetry Enthalpy Of Reaction.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube Calorimetry Enthalpy Of Reaction Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It uses devices called calorimeters,. First, you need to be a little careful about whether the experiment was. Calorimetry Enthalpy Of Reaction.
From lessonlistkilderkins.z22.web.core.windows.net
What Is Enthalpy Quizlet Examples Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. First, you need to be a little careful about whether the experiment was done at constant pressure or constant. Calorimetry is a branch of. Calorimetry Enthalpy Of Reaction.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Chemical Energetics (5) Measuring the enthalpy change of Calorimetry Enthalpy Of Reaction Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calculating the enthalpy change of reaction, hrfrom experimental data. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. Measurement of an. Calorimetry Enthalpy Of Reaction.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Calorimetry Enthalpy Of Reaction A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. In this explainer, we will learn how to perform. Calorimetry Enthalpy Of Reaction.
From www.studocu.com
Enthalpy and Calorimetry lesson 4 Enthalpies of Reaction Calorimetry Enthalpy Of Reaction A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in. Calorimetry Enthalpy Of Reaction.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. Calorimetry is a technique used to measure changes in enthalpy of chemical. Calorimetry Enthalpy Of Reaction.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. A calorimeter can be made up of a polystyrene drinking cup, a. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. Calorimetry Enthalpy Of Reaction.
From www.tes.com
Bond Enthalpy OCR A level Chemistry Teaching Resources Calorimetry Enthalpy Of Reaction Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. First, you need to be a little careful about whether the experiment was done at constant pressure or constant. It uses devices called calorimeters,. Calorimetry is a technique used to measure changes in. Calorimetry Enthalpy Of Reaction.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.2 Calorimetry翰林国际教育 Calorimetry Enthalpy Of Reaction Measurement of an enthalpy change. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. In this explainer, we will learn how to perform calorimetry experiments and use. Calorimetry Enthalpy Of Reaction.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. Measurement of an enthalpy change. A calorimeter can be made up of a polystyrene drinking cup, a.. Calorimetry Enthalpy Of Reaction.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry Enthalpy Of Reaction To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. First, you need to be a little careful about whether the experiment was done at constant pressure or. Calorimetry Enthalpy Of Reaction.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Enthalpy Of Reaction Measurement of an enthalpy change. It uses devices called calorimeters,. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calculating the enthalpy change of reaction, hrfrom experimental data. First, you need to be a little careful about whether the experiment was done at constant pressure or constant. The thermal energy change accompanying a chemical reaction is. Calorimetry Enthalpy Of Reaction.
From grade12uchem.weebly.com
Calorimetry latest copy of grade 12 U Calorimetry Enthalpy Of Reaction To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. Measurement of an enthalpy change. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. In this explainer, we will learn how to perform. Calorimetry Enthalpy Of Reaction.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimetry Enthalpy Of Reaction In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. Measurement of an enthalpy change. A calorimeter can be made up of a polystyrene drinking cup, a. It uses devices called calorimeters,. Calculating the enthalpy change of reaction, hrfrom experimental data. The thermal energy change accompanying a. Calorimetry Enthalpy Of Reaction.
From www.youtube.com
CHEM 101 Calculating Enthalpy of Solution 2 YouTube Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters,. In this explainer, we will learn how to perform calorimetry experiments. Calorimetry Enthalpy Of Reaction.
From www.vernier.com
Determining the Enthalpy of a Chemical Reaction > Experiment 13 from Calorimetry Enthalpy Of Reaction In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Measurement of an enthalpy change. First, you need to. Calorimetry Enthalpy Of Reaction.
From priaxon.com
What Is Standard Enthalpy Of Formation Of Nh3 Gas Templates Printable Calorimetry Enthalpy Of Reaction Measurement of an enthalpy change. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. It uses devices called calorimeters,. The thermal energy. Calorimetry Enthalpy Of Reaction.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记4.1.4 Determining Enthalpy Change of Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. It uses devices called calorimeters,. Measurement of an enthalpy change. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. To determine the enthalpy, stability, heat. Calorimetry Enthalpy Of Reaction.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Enthalpy Of Reaction First, you need to be a little careful about whether the experiment was done at constant pressure or constant. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. Calorimetry is the. Calorimetry Enthalpy Of Reaction.
From studygenevieve.z13.web.core.windows.net
Calculating Enthalpy Of Reaction Worksheet Calorimetry Enthalpy Of Reaction It uses devices called calorimeters,. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. Calculating the enthalpy change of reaction, hrfrom experimental data. Measurement of an enthalpy change. A calorimeter can. Calorimetry Enthalpy Of Reaction.
From www.youtube.com
Calorimetry & Enthalpy Problems YouTube Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calculating the enthalpy change of reaction, hrfrom experimental data. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or. Calorimetry Enthalpy Of Reaction.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube Calorimetry Enthalpy Of Reaction First, you need to be a little careful about whether the experiment was done at constant pressure or constant. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. Measurement. Calorimetry Enthalpy Of Reaction.
From ayanahcristien.blogspot.com
20+ Calculating Heat Of Reaction From ConstantPressure Calorimetry Calorimetry Enthalpy Of Reaction The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. Measurement of an enthalpy change. It uses devices called calorimeters,. Calorimetry is a technique used to. Calorimetry Enthalpy Of Reaction.
From www.nagwa.com
Question Video Calculating the Enthalpy Change for the Reaction Calorimetry Enthalpy Of Reaction A calorimeter can be made up of a polystyrene drinking cup, a. Measurement of an enthalpy change. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. It uses devices called calorimeters,. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is. Calorimetry Enthalpy Of Reaction.
From ar.inspiredpencil.com
Enthalpy Of Reaction Calorimetry Enthalpy Of Reaction Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. To determine the enthalpy, stability, heat capacity,. Calorimetry Enthalpy Of Reaction.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Calorimetry Enthalpy Of Reaction In this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a chemical. Calculating the enthalpy change of reaction, hrfrom experimental data. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions. Calorimetry is a branch of science concerned with measuring a body’s state in terms of. Calorimetry Enthalpy Of Reaction.
From www.studocu.com
Enthalpy and Calorimetry Enthalpy and Calorimetry CHEMpossible By the Calorimetry Enthalpy Of Reaction To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter can be made up of a polystyrene drinking cup, a. First, you need to be a little careful about whether the experiment was done at constant pressure or constant. It uses devices called calorimeters,. Measurement of an enthalpy change. Calorimetry is a technique. Calorimetry Enthalpy Of Reaction.
From learningcampusfox.z1.web.core.windows.net
Coffee Cup Calorimetry Calculator Calorimetry Enthalpy Of Reaction Measurement of an enthalpy change. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It uses devices called calorimeters,. First, you need to be a little careful about whether the experiment was done at constant pressure or constant. Calculating the enthalpy change of reaction, hrfrom experimental data. A calorimeter can be made up of. Calorimetry Enthalpy Of Reaction.