Copper Ii Chloride And Water Balanced Equation . To enter an ion, specify charge after the compound in curly brackets: Explain the roles of subscripts and coefficients in chemical equations. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Substitute immutable groups in chemical. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. So for each mole of copper. Balance a chemical equation when given the. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. $\begingroup$ the formula gives you the ratio already. How do you write a balanced equation for this. {+3} or {3+} or {3}.
from www.slideserve.com
How do you write a balanced equation for this. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Enter an equation of an ionic chemical equation and press the balance button. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. $\begingroup$ the formula gives you the ratio already. So for each mole of copper. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. The balanced equation will be calculated along with the. To enter an ion, specify charge after the compound in curly brackets: In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is.
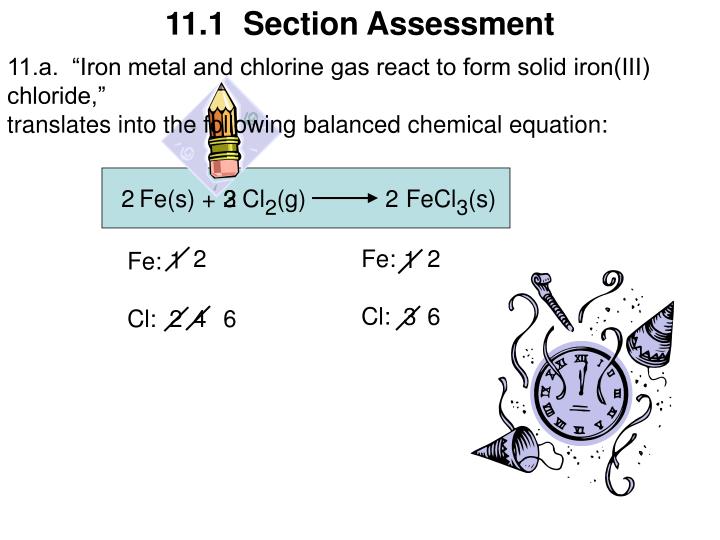
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952
Copper Ii Chloride And Water Balanced Equation When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. Balance a chemical equation when given the. Substitute immutable groups in chemical. Enter an equation of an ionic chemical equation and press the balance button. To enter an ion, specify charge after the compound in curly brackets: So for each mole of copper. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. {+3} or {3+} or {3}. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. The balanced equation will be calculated along with the. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. Explain the roles of subscripts and coefficients in chemical equations. How do you write a balanced equation for this. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. $\begingroup$ the formula gives you the ratio already.
From www.numerade.com
SOLVED If we have 0.572 grams of aluminum and 3.029 grams of copper(II Copper Ii Chloride And Water Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Substitute immutable groups in chemical. To enter an ion, specify charge after the compound in curly brackets: Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. {+3} or {3+} or {3}. In this video we will describe the. Copper Ii Chloride And Water Balanced Equation.
From www.numerade.com
SOLVED If we have 0.572 grams of aluminum and 3.029 grams of copper(II Copper Ii Chloride And Water Balanced Equation The balanced equation will be calculated along with the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. So for each. Copper Ii Chloride And Water Balanced Equation.
From www.numerade.com
Write the balanced molecular equation for each of the following Copper Ii Chloride And Water Balanced Equation So for each mole of copper. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. $\begingroup$. Copper Ii Chloride And Water Balanced Equation.
From www.coursehero.com
[Solved] Please see below question V The names and chemical formulae of Copper Ii Chloride And Water Balanced Equation When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. Enter an equation of an ionic chemical equation and press the balance. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
CuO+HCl=CuCl2+H2O Balanced EquationCopper oxide+Hydrochloric acid Copper Ii Chloride And Water Balanced Equation {+3} or {3+} or {3}. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. Enter an equation of an ionic chemical equation and press the balance button. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In this video we will describe the equation. Copper Ii Chloride And Water Balanced Equation.
From www.coursehero.com
[Solved] copper(II)sulfate + Barium chloride molecular equation and Copper Ii Chloride And Water Balanced Equation How do you write a balanced equation for this. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. $\begingroup$ the formula gives you the ratio already. {+3} or {3+} or {3}. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. Write a balanced equation for the decomposition. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Ii Chloride And Water Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. How do you write a balanced equation for this. Substitute immutable groups in chemical. So for each mole of copper. The balanced equation will be. Copper Ii Chloride And Water Balanced Equation.
From chemicaldb.netlify.app
Is copper chloride a precipitate Copper Ii Chloride And Water Balanced Equation If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. How do you write a balanced equation for this. {+3} or {3+} or {3}. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water. Copper Ii Chloride And Water Balanced Equation.
From www.chegg.com
Solved Mole Ratios Copper(II) chloride (CuCl,; 0.98 g) was Copper Ii Chloride And Water Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. $\begingroup$ the formula gives you the ratio already. Substitute immutable groups in chemical. Balance a chemical equation when given the. The balanced equation will be calculated along with the. Explain the roles of subscripts and coefficients in chemical equations. So for each mole of copper. In this. Copper Ii Chloride And Water Balanced Equation.
From www.pw.live
Copper I Chloride Formula, Structure, Properties, Uses Copper Ii Chloride And Water Balanced Equation The balanced equation will be calculated along with the. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. Substitute immutable groups in chemical. So for each mole of copper. Enter an equation of an. Copper Ii Chloride And Water Balanced Equation.
From www.chegg.com
Solved convert the following word equation into balenced Copper Ii Chloride And Water Balanced Equation In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. Enter an equation of an ionic chemical equation and press the balance button. To enter an ion, specify charge after the compound in curly brackets: The balanced equation will be calculated along with the. Substitute immutable groups in chemical. To balance a. Copper Ii Chloride And Water Balanced Equation.
From parisancerobertson.blogspot.com
Copper Ii Chloride Formula ParisanceRobertson Copper Ii Chloride And Water Balanced Equation $\begingroup$ the formula gives you the ratio already. Substitute immutable groups in chemical. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. To enter an ion, specify charge after the compound in curly brackets: Explain the roles of subscripts and coefficients in chemical equations. {+3} or {3+} or {3}. When sulfuric acid and copper (ii) are. Copper Ii Chloride And Water Balanced Equation.
From www.numerade.com
SOLVED and balance the molecular equation including phases Copper Ii Chloride And Water Balanced Equation So for each mole of copper. Enter an equation of an ionic chemical equation and press the balance button. Substitute immutable groups in chemical. To enter an ion, specify charge after the compound in curly brackets: The balanced equation will be calculated along with the. Balance a chemical equation when given the. {+3} or {3+} or {3}. Write a balanced. Copper Ii Chloride And Water Balanced Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii Chloride And Water Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. So for each mole of copper. Balance a chemical equation when given the. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. {+3} or {3+} or {3}. Substitute immutable groups in chemical. Explain the roles of subscripts and. Copper Ii Chloride And Water Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced chemical equation for each chemical reaction Copper Ii Chloride And Water Balanced Equation To enter an ion, specify charge after the compound in curly brackets: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. So for each mole of copper. How do you write a balanced equation for this. $\begingroup$ the formula gives you the ratio already. If you assume a molecule $\ce{cucl2}$ there is. Copper Ii Chloride And Water Balanced Equation.
From studylib.net
Laboratory 5 Stoichiometry of Copper (II) Chloride and Aluminum Copper Ii Chloride And Water Balanced Equation {+3} or {3+} or {3}. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. To enter an ion, specify charge after the compound in curly brackets: In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. How do you write a balanced equation for this. Explain the roles. Copper Ii Chloride And Water Balanced Equation.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Copper Ii Chloride And Water Balanced Equation The balanced equation will be calculated along with the. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. Substitute immutable groups in chemical. Enter an equation of an ionic chemical equation and press the. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
The electrolysis of copper (II) chloride. YouTube Copper Ii Chloride And Water Balanced Equation If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. To enter an ion, specify charge after the compound in curly brackets:. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
Cu + H2SO4 (Copper + Sulfuric acid) YouTube Copper Ii Chloride And Water Balanced Equation If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. $\begingroup$ the formula gives you the ratio already. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Substitute immutable groups in chemical. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are. Copper Ii Chloride And Water Balanced Equation.
From www.chegg.com
Solved Copper Ii Chloride And Water Balanced Equation So for each mole of copper. Explain the roles of subscripts and coefficients in chemical equations. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Balance a chemical equation when given the. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. How do. Copper Ii Chloride And Water Balanced Equation.
From spmscience.blog.onlinetuition.com.my
Electrolysis of Copper (II) Chloride Solution SPM Science Copper Ii Chloride And Water Balanced Equation How do you write a balanced equation for this. The balanced equation will be calculated along with the. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. Balance a chemical equation when given the.. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for CuCl2 + NaOH = Cu(OH)2 + NaCl Copper Ii Chloride And Water Balanced Equation Balance a chemical equation when given the. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. The balanced equation will be calculated along with the. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. Enter an equation of an ionic chemical equation and. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
How to Balance CuCl2 + NaOH = Cu(OH)2 + NaCl Copper (II) chloride Copper Ii Chloride And Water Balanced Equation Substitute immutable groups in chemical. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. The balanced equation will be calculated along with the. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. Enter an equation of an ionic chemical equation and press the balance button. Balance a. Copper Ii Chloride And Water Balanced Equation.
From ar.inspiredpencil.com
Copper Ii Chloride Lewis Structure Copper Ii Chloride And Water Balanced Equation If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. How do you write a balanced equation for this. Substitute immutable groups in chemical. Enter an equation of an ionic chemical equation and press the balance button. {+3} or {3+}. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
How to Balance Zn + CuCl2 = ZnCl2 + Cu Zinc + Copper (II) chloride Copper Ii Chloride And Water Balanced Equation {+3} or {3+} or {3}. The balanced equation will be calculated along with the. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. So for each mole of copper. Balance a chemical equation when given the. How do you write a balanced equation for this. In this video we will describe. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper Ii Chloride And Water Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. Write a balanced equation for the decomposition of. Copper Ii Chloride And Water Balanced Equation.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Copper Ii Chloride And Water Balanced Equation In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. To enter an ion, specify charge after the compound in curly brackets: {+3} or {3+} or {3}. Explain the roles of subscripts and coefficients in chemical equations. How do you write a balanced equation for this. Enter an equation of an ionic. Copper Ii Chloride And Water Balanced Equation.
From exobqbaxs.blob.core.windows.net
Copper Chloride Reaction With Water at Florence McIntosh blog Copper Ii Chloride And Water Balanced Equation Balance a chemical equation when given the. Substitute immutable groups in chemical. To enter an ion, specify charge after the compound in curly brackets: Explain the roles of subscripts and coefficients in chemical equations. {+3} or {3+} or {3}. How do you write a balanced equation for this. When sulfuric acid and copper (ii) are allowed to react, copper (ii). Copper Ii Chloride And Water Balanced Equation.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952 Copper Ii Chloride And Water Balanced Equation So for each mole of copper. How do you write a balanced equation for this. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. {+3} or {3+} or {3}. $\begingroup$ the formula gives. Copper Ii Chloride And Water Balanced Equation.
From oneclass.com
OneClass Write the balanced net ionic equation for the reactions that Copper Ii Chloride And Water Balanced Equation Balance a chemical equation when given the. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. When sulfuric acid and copper (ii) are allowed to react, copper (ii) sulfate and water are formed. Explain the roles of subscripts and coefficients in chemical equations. Substitute immutable groups in chemical. To balance a chemical equation, enter an equation. Copper Ii Chloride And Water Balanced Equation.
From www.pw.live
Copper II Chloride Formula, Structure, Properties, Uses Copper Ii Chloride And Water Balanced Equation How do you write a balanced equation for this. In this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is. The balanced equation will be calculated along with the. Explain the roles of subscripts and coefficients in chemical equations. To enter an ion, specify charge after the compound in curly brackets: Enter an. Copper Ii Chloride And Water Balanced Equation.
From hxelaptcw.blob.core.windows.net
Copper Ii Sulfate Sodium Phosphate Balanced Equation at Raymond Horan blog Copper Ii Chloride And Water Balanced Equation So for each mole of copper. Explain the roles of subscripts and coefficients in chemical equations. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. {+3} or {3+} or {3}. $\begingroup$ the formula gives you the ratio already. How. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
Electrolysis of concentrated copper (II) chloride (aq) CuCL2 GCSE Copper Ii Chloride And Water Balanced Equation The balanced equation will be calculated along with the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balance a chemical equation when given the. Explain the roles of subscripts and coefficients in chemical equations. Enter an equation of an ionic chemical equation and press the balance button. In this video we. Copper Ii Chloride And Water Balanced Equation.
From exopnayqj.blob.core.windows.net
Silver Nitrate Copper Wire Balanced Equation at Maria Samuels blog Copper Ii Chloride And Water Balanced Equation $\begingroup$ the formula gives you the ratio already. Substitute immutable groups in chemical. Balance a chemical equation when given the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. {+3} or {3+} or {3}. If you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. Explain the roles of subscripts. Copper Ii Chloride And Water Balanced Equation.
From www.youtube.com
Easy tips to balance Aluminium+ copper chloride= Aluminium chloride Copper Ii Chloride And Water Balanced Equation $\begingroup$ the formula gives you the ratio already. How do you write a balanced equation for this. To enter an ion, specify charge after the compound in curly brackets: Substitute immutable groups in chemical. Balance a chemical equation when given the. The balanced equation will be calculated along with the. When sulfuric acid and copper (ii) are allowed to react,. Copper Ii Chloride And Water Balanced Equation.