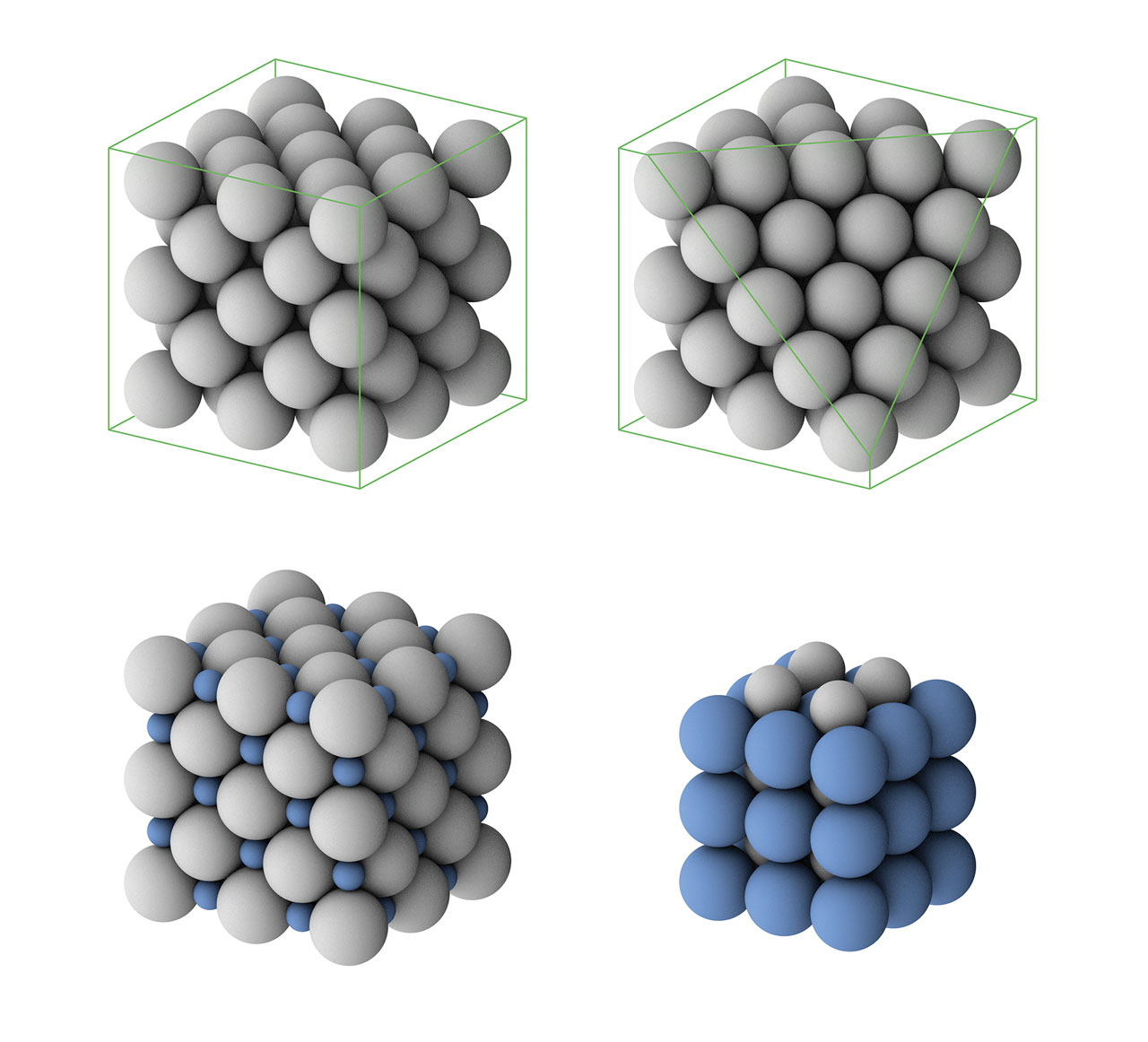
Crystals Definition In Chemistry . A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Because there are repeated units, crystals have recognizable structures. Identify the three kinds of rotational symmetry axes of a cube. There are 4 types of crystals: Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Metallic, ionic, covalent, and molecular. Graphite in pencils, table salt,. Metallic crystals have metallic bonds. But those are not the only types of crystals. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. Crystals are solids in which all of the atoms are arranged in repeating patterns. The lattice that forms extends out in three dimensions. State what is meant by a crystal's habit, and. When we hear the word crystals, we usually think of coloured minerals.
from www.beautifulchemistry.net
When we hear the word crystals, we usually think of coloured minerals. Metallic crystals have metallic bonds. Crystals are solids in which all of the atoms are arranged in repeating patterns. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. But those are not the only types of crystals. Graphite in pencils, table salt,. Because there are repeated units, crystals have recognizable structures. The lattice that forms extends out in three dimensions. There are 4 types of crystals: Identify the three kinds of rotational symmetry axes of a cube.
Crystal Structure — Beautiful Chemistry
Crystals Definition In Chemistry Identify the three kinds of rotational symmetry axes of a cube. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. There are 4 types of crystals: State what is meant by a crystal's habit, and. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. Metallic crystals have metallic bonds. Crystals are solids in which all of the atoms are arranged in repeating patterns. Graphite in pencils, table salt,. Metallic, ionic, covalent, and molecular. The lattice that forms extends out in three dimensions. Identify the three kinds of rotational symmetry axes of a cube. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Because there are repeated units, crystals have recognizable structures. But those are not the only types of crystals. When we hear the word crystals, we usually think of coloured minerals.
From www.geologyin.com
Crystal Structure and Crystal Systems Crystals Definition In Chemistry But those are not the only types of crystals. Crystals are solids in which all of the atoms are arranged in repeating patterns. Metallic, ionic, covalent, and molecular. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Metallic crystals have metallic bonds. There are 4 types. Crystals Definition In Chemistry.
From www.slideserve.com
PPT Chapter 1 Crystal Structure PowerPoint Presentation, free Crystals Definition In Chemistry Crystals are solids in which all of the atoms are arranged in repeating patterns. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. State what is meant by a crystal's habit, and. The lattice that forms extends out in three dimensions. Metallic crystals have metallic bonds.. Crystals Definition In Chemistry.
From chem.libretexts.org
Crystalline Solid Structures Chemistry LibreTexts Crystals Definition In Chemistry Identify the three kinds of rotational symmetry axes of a cube. State what is meant by a crystal's habit, and. The lattice that forms extends out in three dimensions. Metallic crystals have metallic bonds. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. Crystal, any solid material in which the component atoms are arranged in. Crystals Definition In Chemistry.
From www.thoughtco.com
What Is a Crystal in Chemistry? Crystals Definition In Chemistry Metallic crystals have metallic bonds. The lattice that forms extends out in three dimensions. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Crystals are solids in which all of the atoms are arranged in repeating patterns. State what is meant by a crystal's habit, and.. Crystals Definition In Chemistry.
From chemistry.about.com
Types of Crystals Shapes and Structures Crystals Definition In Chemistry Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. The lattice that forms extends out in three dimensions. But those are not the only types of crystals. State what is meant by a crystal's habit, and. Crystals are classified in general categories, such as insulators, metals,. Crystals Definition In Chemistry.
From chem.libretexts.org
Chapter 12.2 Arrangement of Atoms in Crystals Chemistry LibreTexts Crystals Definition In Chemistry Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Identify the three kinds of rotational symmetry axes of a cube. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystals are solids in which all of the atoms. Crystals Definition In Chemistry.
From www.youtube.com
What are different types of crystals. Solid State Physical Crystals Definition In Chemistry There are 4 types of crystals: Metallic crystals have metallic bonds. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. Identify the three kinds of rotational symmetry axes of a cube. Crystals are solids in which all of the atoms are arranged in repeating patterns. But those are not the only types of crystals. Crystal,. Crystals Definition In Chemistry.
From collegedunia.com
Crystallization Definition, Process, Types & Examples Crystals Definition In Chemistry Because there are repeated units, crystals have recognizable structures. Identify the three kinds of rotational symmetry axes of a cube. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystals are solids in which all of the atoms are arranged in repeating patterns. Metallic crystals have metallic bonds. The lattice that forms. Crystals Definition In Chemistry.
From www.slideserve.com
PPT Fundamentals of crystal chemistry PowerPoint Presentation, free Crystals Definition In Chemistry The lattice that forms extends out in three dimensions. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. But those are not the only types of crystals. Because there are repeated units, crystals have recognizable structures. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. Metallic crystals. Crystals Definition In Chemistry.
From byjus.com
Crystal Structure Definition, 7 Types of Crystal Structure with Videos Crystals Definition In Chemistry Identify the three kinds of rotational symmetry axes of a cube. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. When we hear the word crystals, we usually think of coloured minerals. Crystals are solids in which all of the atoms are arranged in repeating patterns. Metallic crystals have metallic bonds. But those are not. Crystals Definition In Chemistry.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Crystals Definition In Chemistry Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Identify the three kinds of rotational symmetry axes of a cube. State what is meant by a crystal's habit, and. There are 4 types of crystals: Because there are repeated units, crystals have recognizable structures. Metallic, ionic,. Crystals Definition In Chemistry.
From www.slideserve.com
PPT Introduction to Minerals PowerPoint Presentation, free download Crystals Definition In Chemistry Metallic, ionic, covalent, and molecular. Metallic crystals have metallic bonds. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. But those are not the only types of crystals. Crystals. Crystals Definition In Chemistry.
From www.ck12.org
Comparing Crystals Overview ( Video ) Chemistry CK12 Foundation Crystals Definition In Chemistry When we hear the word crystals, we usually think of coloured minerals. But those are not the only types of crystals. Because there are repeated units, crystals have recognizable structures. Identify the three kinds of rotational symmetry axes of a cube. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. A crystal consists of matter. Crystals Definition In Chemistry.
From www.beautifulchemistry.net
Crystal Structure — Beautiful Chemistry Crystals Definition In Chemistry Because there are repeated units, crystals have recognizable structures. Metallic, ionic, covalent, and molecular. Crystals are solids in which all of the atoms are arranged in repeating patterns. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. State what is meant by a crystal's habit, and.. Crystals Definition In Chemistry.
From chemistnotes.com
Covalent crystals, Definition, Example, and Properties Chemistry Notes Crystals Definition In Chemistry There are 4 types of crystals: Identify the three kinds of rotational symmetry axes of a cube. Graphite in pencils, table salt,. But those are not the only types of crystals. Crystals are solids in which all of the atoms are arranged in repeating patterns. Crystal, any solid material in which the component atoms are arranged in a definite pattern. Crystals Definition In Chemistry.
From www.youtube.com
Law of crystal symmetry Solid State Physical Chemistry YouTube Crystals Definition In Chemistry Identify the three kinds of rotational symmetry axes of a cube. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. Crystals are solids in which all of the atoms are arranged in repeating patterns. Graphite in pencils, table salt,. Crystal, any solid material in which the component atoms are arranged in a definite pattern and. Crystals Definition In Chemistry.
From www.beautifulchemistry.net
Crystal Structure — Beautiful Chemistry Crystals Definition In Chemistry Crystals are solids in which all of the atoms are arranged in repeating patterns. But those are not the only types of crystals. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects. Crystals Definition In Chemistry.
From www.youtube.com
Unit 1.3 Definition of Crystals and Anisotropy YouTube Crystals Definition In Chemistry But those are not the only types of crystals. There are 4 types of crystals: Identify the three kinds of rotational symmetry axes of a cube. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. The lattice that forms extends out in three dimensions. Metallic crystals have metallic bonds. Crystal, any solid. Crystals Definition In Chemistry.
From www.britannica.com
Crystallography Definition & Facts Britannica Crystals Definition In Chemistry Because there are repeated units, crystals have recognizable structures. There are 4 types of crystals: When we hear the word crystals, we usually think of coloured minerals. Metallic, ionic, covalent, and molecular. Crystals are solids in which all of the atoms are arranged in repeating patterns. But those are not the only types of crystals. The lattice that forms extends. Crystals Definition In Chemistry.
From www.slideserve.com
PPT Lecture 3 Crystal Chemistry Part 2 Bonding and Ionic Radii Crystals Definition In Chemistry A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. There are 4 types of crystals: When we hear the word crystals, we usually think of coloured minerals. Metallic crystals have metallic bonds. Graphite in pencils, table salt,. Metallic, ionic, covalent, and molecular. Crystal, any solid material in which the component atoms are. Crystals Definition In Chemistry.
From www.thoughtco.com
Crystal Definition, Examples, and Common Types Crystals Definition In Chemistry When we hear the word crystals, we usually think of coloured minerals. State what is meant by a crystal's habit, and. Because there are repeated units, crystals have recognizable structures. Identify the three kinds of rotational symmetry axes of a cube. Metallic crystals have metallic bonds. A crystal consists of matter that is formed from an ordered arrangement of atoms,. Crystals Definition In Chemistry.
From opengeology.org
13 Crystal Structures Mineralogy Crystals Definition In Chemistry Metallic crystals have metallic bonds. But those are not the only types of crystals. State what is meant by a crystal's habit, and. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. There are 4 types of crystals: Because there are repeated units, crystals have recognizable. Crystals Definition In Chemistry.
From courses.lumenlearning.com
Types of Crystals Boundless Chemistry Crystals Definition In Chemistry Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. The lattice that forms extends out in three dimensions. Identify the three kinds of rotational symmetry axes of a cube. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Metallic crystals have. Crystals Definition In Chemistry.
From www.slideserve.com
PPT Introduction to Crystallization Chemistry PowerPoint Presentation Crystals Definition In Chemistry Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. When we hear the word crystals, we usually think of coloured minerals. Crystals are solids in which all of the atoms are arranged in repeating patterns. Metallic, ionic, covalent, and molecular. The lattice that forms extends out. Crystals Definition In Chemistry.
From chemistnotes.com
Covalent crystals, Definition, Example, and Properties Chemistry Notes Crystals Definition In Chemistry Metallic, ionic, covalent, and molecular. Metallic crystals have metallic bonds. Crystals are solids in which all of the atoms are arranged in repeating patterns. But those are not the only types of crystals. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Graphite in pencils, table salt,. Crystal, any solid material in. Crystals Definition In Chemistry.
From chem.libretexts.org
12.6 Crystal Structures Chemistry LibreTexts Crystals Definition In Chemistry A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Because there are repeated units, crystals have recognizable structures. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. But those are not the only types of crystals. When we hear the word crystals, we usually think of coloured. Crystals Definition In Chemistry.
From gamesmartz.com
Crystal Definition & Image GameSmartz Crystals Definition In Chemistry Because there are repeated units, crystals have recognizable structures. Crystals are solids in which all of the atoms are arranged in repeating patterns. There are 4 types of crystals: Metallic crystals have metallic bonds. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. State what is. Crystals Definition In Chemistry.
From www.slideserve.com
PPT Lecture 2 (9/11/2006) Crystal Chemistry Part 1 Atoms, Elements Crystals Definition In Chemistry Because there are repeated units, crystals have recognizable structures. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. But those are not the only types of crystals. Crystals are solids in which all of the atoms are arranged in repeating patterns. When we hear the word. Crystals Definition In Chemistry.
From www.britannica.com
chemical bonding Definition, Types, & Examples Britannica Crystals Definition In Chemistry Metallic crystals have metallic bonds. Graphite in pencils, table salt,. Crystals are solids in which all of the atoms are arranged in repeating patterns. State what is meant by a crystal's habit, and. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystals are classified in general categories, such as insulators, metals,. Crystals Definition In Chemistry.
From samson-blogjenkins.blogspot.com
Measurements Used to Describe Crystal Structures Crystals Definition In Chemistry Metallic crystals have metallic bonds. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Identify the three kinds of rotational symmetry axes of a cube. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. There are 4 types of crystals: The lattice that forms extends out in. Crystals Definition In Chemistry.
From chem.libretexts.org
12.1 Crystalline and Amorphous Solids Chemistry LibreTexts Crystals Definition In Chemistry But those are not the only types of crystals. Metallic, ionic, covalent, and molecular. Crystals are classified in general categories, such as insulators, metals, semiconductors, and molecular solids. Identify the three kinds of rotational symmetry axes of a cube. The lattice that forms extends out in three dimensions. Because there are repeated units, crystals have recognizable structures. There are 4. Crystals Definition In Chemistry.
From www.slideserve.com
PPT Lecture 3 Rocks and Minerals PowerPoint Presentation, free Crystals Definition In Chemistry A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystals are solids in which all of the atoms are arranged in repeating patterns. Metallic, ionic, covalent, and molecular. Graphite in pencils, table salt,. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity. Crystals Definition In Chemistry.
From courses.lumenlearning.com
Types of Crystals Boundless Chemistry Crystals Definition In Chemistry A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. When we hear the word crystals, we usually think of coloured minerals. Metallic crystals have metallic bonds. But those are not the only types of crystals. Identify the three kinds of rotational symmetry axes of a cube. Crystal, any solid material in which. Crystals Definition In Chemistry.
From www.alamy.com
blue, copper, crystals, chemistry, cobalt, sulphate, crystallization Crystals Definition In Chemistry Graphite in pencils, table salt,. Crystals are solids in which all of the atoms are arranged in repeating patterns. But those are not the only types of crystals. The lattice that forms extends out in three dimensions. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry.. Crystals Definition In Chemistry.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Crystals Definition In Chemistry But those are not the only types of crystals. State what is meant by a crystal's habit, and. Identify the three kinds of rotational symmetry axes of a cube. Metallic crystals have metallic bonds. Crystals are solids in which all of the atoms are arranged in repeating patterns. Graphite in pencils, table salt,. The lattice that forms extends out in. Crystals Definition In Chemistry.