Zinc Electron Quantum Numbers . Magnetic quantum number (m l): It has an atomic weight of 65.38 and a mass. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. Spin quantum number (m s ): Electron configuration chart of all elements is mentioned in the table below. Indicates the orientation of the orbital in space. A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The shorthand electron configuration (or noble gas configuration) as well as. N, l, m l, and m s. N determines the general range for the value of energy. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Describe the properties of an electron associated with each of the following four quantum numbers:
from www.alamy.es
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. Magnetic quantum number (m l): Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Spin quantum number (m s ): The shorthand electron configuration (or noble gas configuration) as well as. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. Describe the properties of an electron associated with each of the following four quantum numbers: N, l, m l, and m s. N determines the general range for the value of energy. Electron configuration chart of all elements is mentioned in the table below.
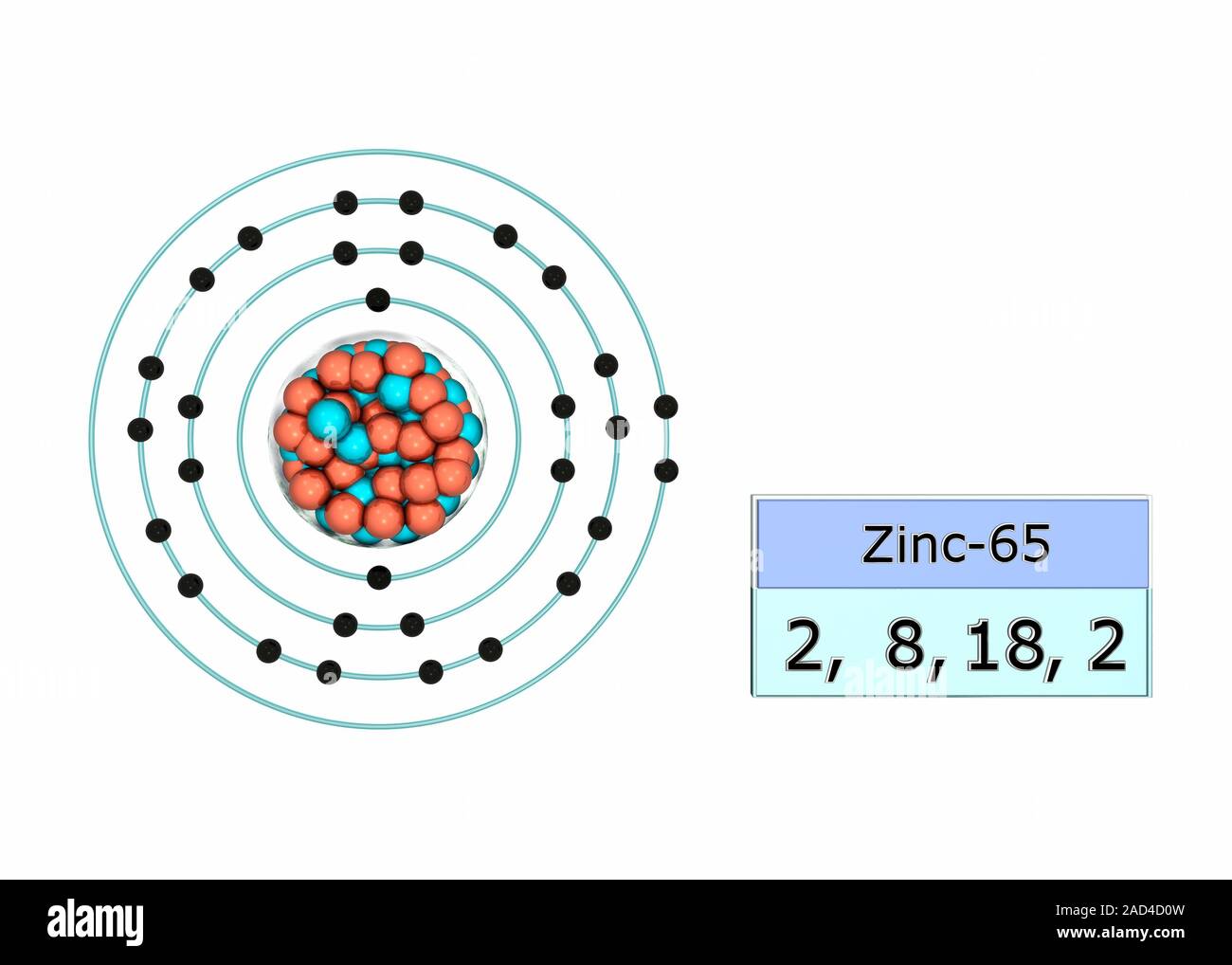
Configuración de electrones del zinc. Ilustración de la estructura
Zinc Electron Quantum Numbers Electron configuration chart of all elements is mentioned in the table below. A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. It has an atomic weight of 65.38 and a mass. Indicates the orientation of the orbital in space. Describe the properties of an electron associated with each of the following four quantum numbers: The shorthand electron configuration (or noble gas configuration) as well as. N determines the general range for the value of energy. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. N, l, m l, and m s. Magnetic quantum number (m l): Spin quantum number (m s ): Electron configuration chart of all elements is mentioned in the table below.
From valenceelectrons.com
Protons, Neutrons, Electrons for Zinc (Zn, Zn2+) Zinc Electron Quantum Numbers N, l, m l, and m s. It has an atomic weight of 65.38 and a mass. Indicates the orientation of the orbital in space. N determines the general range for the value of energy. Magnetic quantum number (m l): Electron configuration chart of all elements is mentioned in the table below. A total of four quantum numbers are used. Zinc Electron Quantum Numbers.
From www.numerade.com
SOLVED Consider the ground state of zinc, Zn. How many electrons have Zinc Electron Quantum Numbers Indicates the orientation of the orbital in space. It has an atomic weight of 65.38 and a mass. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Describe the properties of an electron associated with each of the following four quantum numbers: N determines the general range for the. Zinc Electron Quantum Numbers.
From valenceelectrons.com
How to Find the Valence Electrons for Carbon(C)? Zinc Electron Quantum Numbers N determines the general range for the value of energy. Magnetic quantum number (m l): Describe the properties of an electron associated with each of the following four quantum numbers: Electron configuration chart of all elements is mentioned in the table below. It has an atomic weight of 65.38 and a mass. The shorthand electron configuration (or noble gas configuration). Zinc Electron Quantum Numbers.
From www.slideserve.com
PPT Ch. 7 Atomic and Electronic Structure PowerPoint Presentation Zinc Electron Quantum Numbers The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. Electron configuration chart of all elements is mentioned in the table below. Indicates the orientation of the orbital in space. Spin quantum number (m s ): N determines the general range for the value. Zinc Electron Quantum Numbers.
From www.freepik.com
Free Vector A zinc atom diagram Zinc Electron Quantum Numbers Electron configuration chart of all elements is mentioned in the table below. N determines the general range for the value of energy. Describe the properties of an electron associated with each of the following four quantum numbers: The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is. Zinc Electron Quantum Numbers.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps Zinc Electron Quantum Numbers Electron configuration chart of all elements is mentioned in the table below. N determines the general range for the value of energy. A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. Spin quantum number (m s ): It has an atomic weight of 65.38 and a mass. Magnetic. Zinc Electron Quantum Numbers.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Zinc (Zn Zinc Electron Quantum Numbers The shorthand electron configuration (or noble gas configuration) as well as. Describe the properties of an electron associated with each of the following four quantum numbers: Spin quantum number (m s ): Magnetic quantum number (m l): Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. The complete electron. Zinc Electron Quantum Numbers.
From valenceelectrons.com
How to Find the Valence Electrons for Zinc (Zn)? Zinc Electron Quantum Numbers A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. Describe the properties of an electron associated with each of the following four quantum numbers: It has an atomic weight of 65.38 and a mass. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2. Zinc Electron Quantum Numbers.
From www.youtube.com
zinc electronic configuration How to Write Zinc electronic Zinc Electron Quantum Numbers Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Describe the properties of an electron associated with each of the following four quantum numbers: Spin quantum number (m s ): The shorthand electron configuration (or noble gas configuration) as well as. Indicates the orientation of the orbital in space.. Zinc Electron Quantum Numbers.
From en.ppt-online.org
Atomic structure and properties. (Chapter 3) online presentation Zinc Electron Quantum Numbers Magnetic quantum number (m l): The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. N determines the general range for the value of energy. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of. Zinc Electron Quantum Numbers.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Zinc Electron Quantum Numbers N, l, m l, and m s. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. It has an atomic weight of 65.38 and a mass. Magnetic quantum number (m l): Spin quantum number (m s ): The shorthand electron configuration (or noble gas configuration) as well as. The. Zinc Electron Quantum Numbers.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Electron Quantum Numbers Spin quantum number (m s ): Electron configuration chart of all elements is mentioned in the table below. Magnetic quantum number (m l): The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. N determines the general range for the value of energy. A. Zinc Electron Quantum Numbers.
From www.alamy.es
Configuración de electrones del zinc. Ilustración de la estructura Zinc Electron Quantum Numbers It has an atomic weight of 65.38 and a mass. N, l, m l, and m s. The shorthand electron configuration (or noble gas configuration) as well as. Spin quantum number (m s ): Electron configuration chart of all elements is mentioned in the table below. Indicates the orientation of the orbital in space. Describe the properties of an electron. Zinc Electron Quantum Numbers.
From physicscatalyst.com
Quantum Numbers Chart physicscatalyst's Blog Zinc Electron Quantum Numbers N determines the general range for the value of energy. It has an atomic weight of 65.38 and a mass. Spin quantum number (m s ): A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. Magnetic quantum number (m l): Zinc is the 30th element in the periodic. Zinc Electron Quantum Numbers.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Zinc Electron Quantum Numbers It has an atomic weight of 65.38 and a mass. The shorthand electron configuration (or noble gas configuration) as well as. Indicates the orientation of the orbital in space. Describe the properties of an electron associated with each of the following four quantum numbers: Magnetic quantum number (m l): N, l, m l, and m s. A total of four. Zinc Electron Quantum Numbers.
From www.youtube.com
The number of electrons for `Zn^(2+)` cation that have the value of Zinc Electron Quantum Numbers A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. Electron configuration chart of all elements is mentioned in the table below. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Indicates the orientation of the orbital in. Zinc Electron Quantum Numbers.
From www.alamy.com
Symbol and electron diagram for Zinc illustration Stock Vector Image Zinc Electron Quantum Numbers N determines the general range for the value of energy. A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The shorthand electron configuration (or noble gas configuration) as well as. Magnetic quantum number (m l): Indicates the orientation of the orbital in space. It has an atomic weight. Zinc Electron Quantum Numbers.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation Zinc Electron Quantum Numbers N, l, m l, and m s. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. N determines the general range for the value of energy. Electron configuration chart of all elements is mentioned in the table below. Describe the properties of an. Zinc Electron Quantum Numbers.
From www.nuclear-power.com
Zinc Electron Affinity Electronegativity Ionization Energy of Zinc Electron Quantum Numbers Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. It has an atomic weight of 65.38 and a mass. A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. Indicates the orientation of the orbital in space. Describe. Zinc Electron Quantum Numbers.
From www.youtube.com
Electron Configuration of Zinc Zn Lesson YouTube Zinc Electron Quantum Numbers Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Indicates the orientation of the orbital in space. Describe the properties of an electron associated with each of the following four quantum numbers: Spin quantum number (m s ): N determines the general range for the value of energy. It. Zinc Electron Quantum Numbers.
From askfilo.com
Electronic Configuration of Scandium (Z=21) to Zinc (Z=30) In these ele.. Zinc Electron Quantum Numbers Spin quantum number (m s ): The shorthand electron configuration (or noble gas configuration) as well as. It has an atomic weight of 65.38 and a mass. Describe the properties of an electron associated with each of the following four quantum numbers: Indicates the orientation of the orbital in space. Electron configuration chart of all elements is mentioned in the. Zinc Electron Quantum Numbers.
From www.numerade.com
VIDEO solution Give the set of four quantum numbers that represent the Zinc Electron Quantum Numbers The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. The shorthand electron configuration (or noble gas configuration) as well as. N, l, m l, and m s. Spin quantum number (m s ): A total of four quantum numbers are used to describe. Zinc Electron Quantum Numbers.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Quantum Numbers Indicates the orientation of the orbital in space. Spin quantum number (m s ): The shorthand electron configuration (or noble gas configuration) as well as. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. It has an atomic weight of 65.38 and a. Zinc Electron Quantum Numbers.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Quantum Numbers Magnetic quantum number (m l): Indicates the orientation of the orbital in space. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. N, l, m l, and m s. N determines the general range for the value of energy. Describe the properties of. Zinc Electron Quantum Numbers.
From manuallistcantabank.z21.web.core.windows.net
Zinc Orbital Diagram Zinc Electron Quantum Numbers Spin quantum number (m s ): Indicates the orientation of the orbital in space. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in. Zinc Electron Quantum Numbers.
From www.alamy.com
Zinc (Zn). Diagram of the nuclear composition, electron configuration Zinc Electron Quantum Numbers It has an atomic weight of 65.38 and a mass. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. N determines the general range for the value of energy. Indicates the orientation of the orbital in space. Spin quantum number (m s ): Electron configuration chart of all elements. Zinc Electron Quantum Numbers.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Electron Quantum Numbers N, l, m l, and m s. N determines the general range for the value of energy. It has an atomic weight of 65.38 and a mass. The shorthand electron configuration (or noble gas configuration) as well as. Describe the properties of an electron associated with each of the following four quantum numbers: Electron configuration chart of all elements is. Zinc Electron Quantum Numbers.
From www.chemistrylearner.com
Zinc Facts, Symbol, Discovery, Properties, Uses Zinc Electron Quantum Numbers The shorthand electron configuration (or noble gas configuration) as well as. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. N determines the general range for the value of energy. Describe the properties of an electron associated with each of the following four quantum numbers: Indicates the orientation of. Zinc Electron Quantum Numbers.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Quantum Numbers Electron configuration chart of all elements is mentioned in the table below. Describe the properties of an electron associated with each of the following four quantum numbers: Indicates the orientation of the orbital in space. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. A total of four quantum. Zinc Electron Quantum Numbers.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Quantum Numbers A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. Spin quantum number (m s ): Indicates the orientation of the orbital in space. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10. Zinc Electron Quantum Numbers.
From valenceelectrons.com
How to Write the Electron Configuration for Zinc (Zn)? Zinc Electron Quantum Numbers It has an atomic weight of 65.38 and a mass. N, l, m l, and m s. Magnetic quantum number (m l): Spin quantum number (m s ): A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. Indicates the orientation of the orbital in space. N determines the. Zinc Electron Quantum Numbers.
From www.schoolmykids.com
Zinc (Zn) Element Information, Facts, Properties, Uses Periodic Zinc Electron Quantum Numbers Indicates the orientation of the orbital in space. The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. Magnetic quantum number (m l): It has an atomic weight of 65.38 and a mass. Spin quantum number (m s ): Describe the properties of an. Zinc Electron Quantum Numbers.
From ar.inspiredpencil.com
Zinc Electron Configuration Zinc Electron Quantum Numbers Electron configuration chart of all elements is mentioned in the table below. Describe the properties of an electron associated with each of the following four quantum numbers: Indicates the orientation of the orbital in space. The shorthand electron configuration (or noble gas configuration) as well as. A total of four quantum numbers are used to describe completely the movement and. Zinc Electron Quantum Numbers.
From ar.inspiredpencil.com
Quantum Numbers Zinc Electron Quantum Numbers Describe the properties of an electron associated with each of the following four quantum numbers: It has an atomic weight of 65.38 and a mass. Electron configuration chart of all elements is mentioned in the table below. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. The shorthand electron. Zinc Electron Quantum Numbers.
From periodictableguide.com
Zinc (Zn) Periodic Table (Element Information & More) Zinc Electron Quantum Numbers The complete electron configuration for zinc should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and the abbreviated electron configuration is [ar] 3d10 4s2. Describe the properties of an electron associated with each of the following four quantum numbers: Electron configuration chart of all elements is mentioned in the table below. Spin quantum number (m s ): A. Zinc Electron Quantum Numbers.