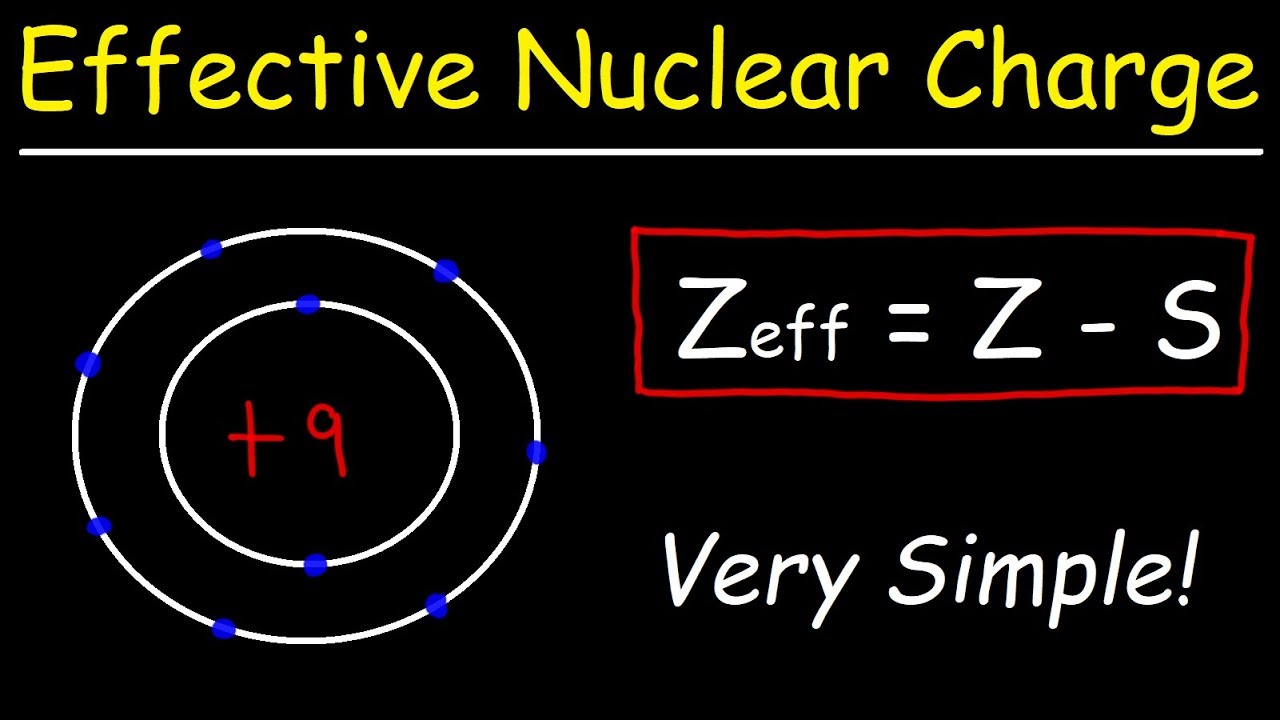
Electron Shielding Of Nuclear Charge . effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. It can be approximated by the equation: the effective nuclear charge is the net positive charge experienced by valence electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an.
from www.youtube.com
the effective nuclear charge is the net positive charge experienced by valence electrons. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. It can be approximated by the equation: the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any.
How To Calculate The Effective Nuclear Charge of an Electron YouTube
Electron Shielding Of Nuclear Charge the effective nuclear charge is the net positive charge experienced by valence electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. the effective nuclear charge is the net positive charge experienced by valence electrons. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. It can be approximated by the equation:
From slideplayer.com
Periodic Trends. ppt download Electron Shielding Of Nuclear Charge It can be approximated by the equation: Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. the effective nuclear charge is the net positive. Electron Shielding Of Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube Electron Shielding Of Nuclear Charge the effective nuclear charge is the net positive charge experienced by valence electrons. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. It can be approximated by the equation: . Electron Shielding Of Nuclear Charge.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive Electron Shielding Of Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. the effective nuclear charge is the net positive charge experienced by valence electrons. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. the concept of electron shielding, in which. Electron Shielding Of Nuclear Charge.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e Electron Shielding Of Nuclear Charge the effective nuclear charge is the net positive charge experienced by valence electrons. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. It can be approximated by the equation: the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an.. Electron Shielding Of Nuclear Charge.
From wisc.pb.unizin.org
Core and Valence Electrons, Shielding, Zeff (M7Q8) UWMadison Electron Shielding Of Nuclear Charge the effective nuclear charge is the net positive charge experienced by valence electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. It can be approximated by the equation: Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. . Electron Shielding Of Nuclear Charge.
From thechemistrynotes.com
Shielding effect Electron Shielding Of Nuclear Charge the effective nuclear charge is the net positive charge experienced by valence electrons. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. the concept of electron shielding, in which intervening. Electron Shielding Of Nuclear Charge.
From www.youtube.com
nuclear charge and electron shielding YouTube Electron Shielding Of Nuclear Charge in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. the effective nuclear charge is the net positive charge experienced by valence electrons. the concept of electron shielding, in which. Electron Shielding Of Nuclear Charge.
From www.learner.org
Organizing Atoms and Electrons The Periodic Table Annenberg Learner Electron Shielding Of Nuclear Charge the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. It can be approximated by the equation: in this section, we explore one model for quantitatively estimating the impact of electron. Electron Shielding Of Nuclear Charge.
From www.numerade.com
SOLVEDSlater's rules are a simple way to estimate the effective Electron Shielding Of Nuclear Charge the effective nuclear charge is the net positive charge experienced by valence electrons. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. the concept of electron shielding, in which. Electron Shielding Of Nuclear Charge.
From www.toppr.com
The ionization energies from Ga to Tl do not decrease due to Electron Shielding Of Nuclear Charge the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. It can be approximated by the equation: Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear. Electron Shielding Of Nuclear Charge.
From www.nuclear-power.com
Shielding of Neutron Radiation Types & Uses Electron Shielding Of Nuclear Charge in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. the effective nuclear charge is the net positive charge experienced by valence electrons. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. effective nuclear charge, z eff, measures how strongly. Electron Shielding Of Nuclear Charge.
From www.numerade.com
SOLVED If core electrons completely shielded valence electrons from Electron Shielding Of Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. the effective nuclear charge is the net positive charge experienced by valence electrons. the concept of electron shielding, in which. Electron Shielding Of Nuclear Charge.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE Electron Shielding Of Nuclear Charge It can be approximated by the equation: the effective nuclear charge is the net positive charge experienced by valence electrons. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. the. Electron Shielding Of Nuclear Charge.
From educationfruits.z21.web.core.windows.net
What Is Shielding Effect In Chemistry Electron Shielding Of Nuclear Charge the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to. Electron Shielding Of Nuclear Charge.
From perso.numericable.fr
The electrons in the inner shells shield the electrons in the outer Electron Shielding Of Nuclear Charge Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. It can be approximated by the equation: the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear. Electron Shielding Of Nuclear Charge.
From www.shemmassianconsulting.com
Atoms and Periodic Trends for the MCAT Everything You Need to Know Electron Shielding Of Nuclear Charge in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate. Electron Shielding Of Nuclear Charge.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube Electron Shielding Of Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. It can be approximated by the equation: in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. in this section, we explore one model for quantitatively estimating the impact of electron. Electron Shielding Of Nuclear Charge.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity Electron Shielding Of Nuclear Charge the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in this. Electron Shielding Of Nuclear Charge.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Electron Shielding Of Nuclear Charge the effective nuclear charge is the net positive charge experienced by valence electrons. It can be approximated by the equation: in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. the concept of electron shielding, in which intervening electrons act to reduce. Electron Shielding Of Nuclear Charge.
From brokeasshome.com
How To Find Nuclear Charge On Periodic Table Electron Shielding Of Nuclear Charge Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the effective nuclear charge is the net positive charge experienced by valence electrons. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. in this section, we explore one model for. Electron Shielding Of Nuclear Charge.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electron Shielding Of Nuclear Charge Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. the effective. Electron Shielding Of Nuclear Charge.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Electron Shielding Of Nuclear Charge in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron,. Electron Shielding Of Nuclear Charge.
From pt.slideshare.net
Periodic trends Electron Shielding Of Nuclear Charge the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. in atomic physics, the effective nuclear charge is the actual amount of positive. Electron Shielding Of Nuclear Charge.
From www.youtube.com
Effective nuclear charge, shielding effect, power, Slater’s Electron Shielding Of Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. It can be approximated by the equation: in this section, we explore one model for quantitatively estimating the impact of electron shielding,. Electron Shielding Of Nuclear Charge.
From www.youtube.com
How To Calculate The Effective Nuclear Charge of an Electron YouTube Electron Shielding Of Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. It can be approximated by the equation: the concept of electron shielding, in which intervening electrons act to reduce the positive. Electron Shielding Of Nuclear Charge.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 Electron Shielding Of Nuclear Charge the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. the effective nuclear charge is the net positive charge experienced by valence electrons. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge.. Electron Shielding Of Nuclear Charge.
From socratic.org
How are shielding effect and atomic radius related? Socratic Electron Shielding Of Nuclear Charge the effective nuclear charge is the net positive charge experienced by valence electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. Effective nuclear charge, zeff, experienced by an. Electron Shielding Of Nuclear Charge.
From www.breakingatom.com
Nuclear Charge Electron Shielding Of Nuclear Charge in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear. Electron Shielding Of Nuclear Charge.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Electron Shielding Of Nuclear Charge the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. It can be approximated by the equation: effective nuclear charge, z eff, measures. Electron Shielding Of Nuclear Charge.
From chem.libretexts.org
3.2 Shielding Chemistry LibreTexts Electron Shielding Of Nuclear Charge in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge,. Electron Shielding Of Nuclear Charge.
From www.slideserve.com
PPT Quantum Theory PowerPoint Presentation, free download ID1185278 Electron Shielding Of Nuclear Charge in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. the effective nuclear charge is the net positive charge experienced by valence electrons. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. It. Electron Shielding Of Nuclear Charge.
From www.nuclear-power.com
Shielding of Beta Radiation Types & Uses Electron Shielding Of Nuclear Charge It can be approximated by the equation: the effective nuclear charge is the net positive charge experienced by valence electrons. in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. Effective. Electron Shielding Of Nuclear Charge.
From chemisfast.blogspot.com
Lanthanide contractiondefinitioncausesconsequences in chemistry PG Electron Shielding Of Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. It can be approximated by the equation: in atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an. in this section, we explore one model for quantitatively estimating the impact of electron. Electron Shielding Of Nuclear Charge.
From www.breakingatom.com
Nuclear Charge of Atoms Electron Shielding Of Nuclear Charge in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear. Electron Shielding Of Nuclear Charge.
From www.slideserve.com
PPT Chemistry periodicity Atomic and Ionic Radius PowerPoint Electron Shielding Of Nuclear Charge in this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge. Effective nuclear charge, zeff, experienced by an electron is less than the actual nuclear charge, z electrons in. the effective nuclear charge is the net positive charge experienced by valence electrons. It can. Electron Shielding Of Nuclear Charge.