Chlorine Reaction Alkene . draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. 10.4 reactions of alkenes: The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. write the equation for the reaction of chlorine or bromine with a given alkene. Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. figure 8.5a addition reaction: These reactions are usually spontaneous. chlorine, bromine or iodine can be added to an alkene. Identify the conditions under which an addition reaction occurs between an alkene.
from www.masterorganicchemistry.com
Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. figure 8.5a addition reaction: chlorine, bromine or iodine can be added to an alkene. These reactions are usually spontaneous. Identify the conditions under which an addition reaction occurs between an alkene. write the equation for the reaction of chlorine or bromine with a given alkene.
Summary Alkene Reaction Pathways — Master Organic Chemistry
Chlorine Reaction Alkene Identify the conditions under which an addition reaction occurs between an alkene. in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. write the equation for the reaction of chlorine or bromine with a given alkene. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. chlorine, bromine or iodine can be added to an alkene. figure 8.5a addition reaction: Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. These reactions are usually spontaneous. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. 10.4 reactions of alkenes: Identify the conditions under which an addition reaction occurs between an alkene.
From byjus.com
In the free radical chlorination of methane, the chain initiation step Chlorine Reaction Alkene in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. 10.4 reactions of alkenes: The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2. Chlorine Reaction Alkene.
From 9to5science.com
[Solved] Reaction of alcohols with PCl5 and PCl3 9to5Science Chlorine Reaction Alkene figure 8.5a addition reaction: draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. 10.4 reactions of alkenes: write the equation for the reaction of. Chlorine Reaction Alkene.
From www.researchgate.net
Scheme 2. Mechanism calculated of the chlorination reaction with Chlorine Reaction Alkene Identify the conditions under which an addition reaction occurs between an alkene. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. chlorine, bromine or iodine can be added to an alkene. 10.4 reactions of alkenes: in the presence of aprotic solvent, the product. Chlorine Reaction Alkene.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Chlorine Reaction Alkene The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. chlorine, bromine or iodine can be added to an alkene. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. When alkenes ( also known as olefins) are. Chlorine Reaction Alkene.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Chain reaction Chlorine Reaction Alkene figure 8.5a addition reaction: Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. These reactions are usually spontaneous. The alkene halogenation reaction, specifically bromination or chlorination,. Chlorine Reaction Alkene.
From www.masterorganicchemistry.com
Thionyl Chloride (SOCl2) Master Organic Chemistry Chlorine Reaction Alkene Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. 10.4 reactions of alkenes: These reactions are usually spontaneous. write the equation for the reaction of chlorine or bromine with a given alkene. figure 8.5a addition reaction: When alkenes ( also known as olefins) are treated with bromine. Chlorine Reaction Alkene.
From alevelchemistry.co.uk
Reactions of Alkanes ALevel Chemistry Revision Notes Chlorine Reaction Alkene These reactions are usually spontaneous. figure 8.5a addition reaction: in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. write the equation for the reaction of chlorine. Chlorine Reaction Alkene.
From byjus.com
when propene reacts with CL2 in light gives A . A reacts with chlorine Chlorine Reaction Alkene chlorine, bromine or iodine can be added to an alkene. 10.4 reactions of alkenes: write the equation for the reaction of chlorine or bromine with a given alkene. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. Identify the conditions under which an. Chlorine Reaction Alkene.
From www.doubtnut.com
Alkenes undergo a variety of oxidation reactions. With cold and neutral Chlorine Reaction Alkene Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. write the equation for the reaction of chlorine or bromine with a given alkene. These reactions are usually spontaneous. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. figure 8.5a. Chlorine Reaction Alkene.
From www.masterorganicchemistry.com
Summary Alkene Reaction Pathways — Master Organic Chemistry Chlorine Reaction Alkene These reactions are usually spontaneous. figure 8.5a addition reaction: in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. Addition of bromine and chlorine to alkenes an addition reaction. Chlorine Reaction Alkene.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes — Master Organic Chemistry Chlorine Reaction Alkene Identify the conditions under which an addition reaction occurs between an alkene. Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. 10.4 reactions of alkenes: write the equation for the reaction of chlorine or bromine with a given alkene. These reactions are usually spontaneous. in the presence. Chlorine Reaction Alkene.
From www.numerade.com
SOLVEDProvide the structures of the product that ommed the following Chlorine Reaction Alkene Identify the conditions under which an addition reaction occurs between an alkene. figure 8.5a addition reaction: When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. 10.4 reactions of alkenes: draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. write. Chlorine Reaction Alkene.
From www.toppr.com
Chlorobenzene is formed by reaction of chlorine with benzene in the Chlorine Reaction Alkene in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. 10.4 reactions of alkenes: These reactions are usually spontaneous. write the equation for the reaction of. Chlorine Reaction Alkene.
From chemistry-europe.onlinelibrary.wiley.com
The Electrochemical cis‐Chlorination of Alkenes Strehl 2021 Chlorine Reaction Alkene When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. Addition of. Chlorine Reaction Alkene.
From www.cell.com
Reaction Direct chlorination of ethane to dichloroethane Chem Chlorine Reaction Alkene figure 8.5a addition reaction: 10.4 reactions of alkenes: When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. These reactions are usually spontaneous. Addition of bromine and chlorine to alkenes an addition reaction also. Chlorine Reaction Alkene.
From quizlet.com
Chlorination of Methane Diagram Quizlet Chlorine Reaction Alkene chlorine, bromine or iodine can be added to an alkene. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. Identify the conditions under which an addition reaction occurs between an alkene. These reactions are usually spontaneous. in the presence of aprotic solvent, the product is a vicinal dihalide, as. Chlorine Reaction Alkene.
From kpu.pressbooks.pub
10.3 Reactions of Alkenes Addition of Water (or Alcohol) to Alkenes Chlorine Reaction Alkene figure 8.5a addition reaction: Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. 10.4 reactions of alkenes: Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. write the equation for the reaction of. Chlorine Reaction Alkene.
From www.pinterest.ca
Synthesis (4) Alkene Reaction Map, Including Alkyl Halide Reactions Chlorine Reaction Alkene The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. These reactions are usually spontaneous. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. 10.4 reactions of alkenes: chlorine, bromine or iodine can be added to an alkene. Identify the conditions under which. Chlorine Reaction Alkene.
From www.masterorganicchemistry.com
Alkene Reactions Practice Problems Master Organic Chemistry Chlorine Reaction Alkene These reactions are usually spontaneous. Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. figure 8.5a addition reaction: 10.4 reactions of alkenes: When alkenes ( also known as olefins) are. Chlorine Reaction Alkene.
From www.masterorganicchemistry.com
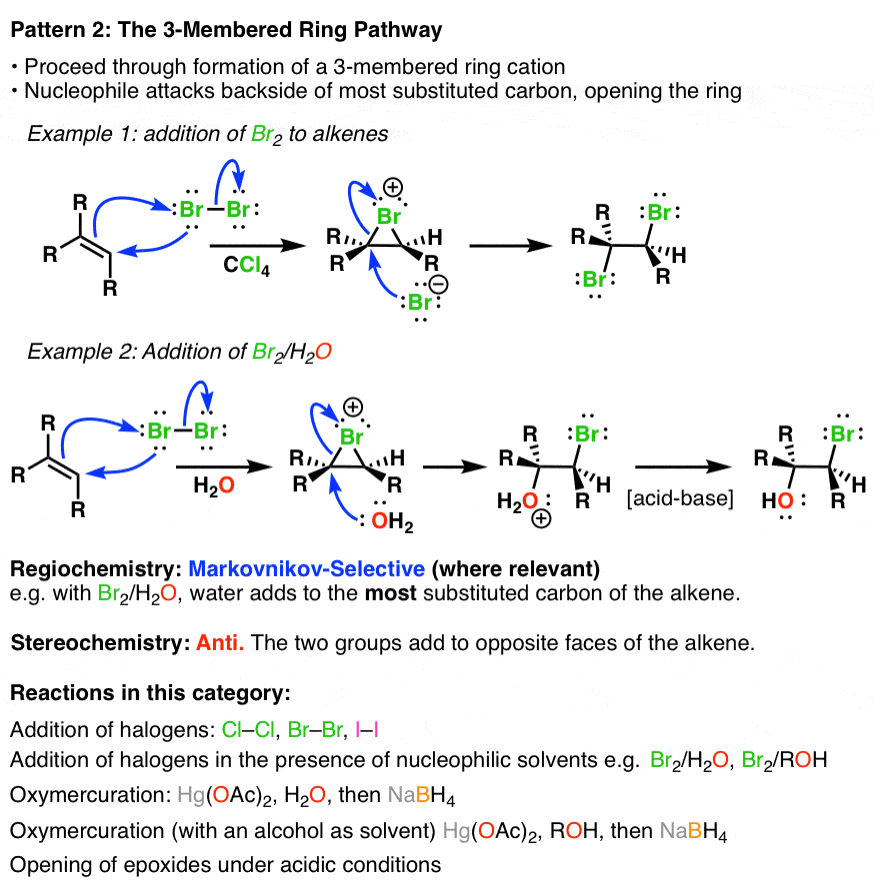
Alkene Addition Pattern 2 The “ThreeMembered Ring” Pathway — Master Chlorine Reaction Alkene Identify the conditions under which an addition reaction occurs between an alkene. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. 10.4 reactions of alkenes: Addition of bromine. Chlorine Reaction Alkene.
From chemtube3d.com
Radicals Chlorination of alkanes Chlorine Reaction Alkene Identify the conditions under which an addition reaction occurs between an alkene. draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. These reactions are usually spontaneous. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. figure 8.5a addition reaction: Addition of. Chlorine Reaction Alkene.
From www.researchgate.net
Scheme 13 Reaction of chlorine dioxide with ketosulfides 4249 Chlorine Reaction Alkene The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. in the. Chlorine Reaction Alkene.
From www.onlinemathlearning.com
Organic Chemistry IGCSE Chemistry (solutions, examples, worksheets Chlorine Reaction Alkene The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to.. Chlorine Reaction Alkene.
From www.chegg.com
Solved Consider the mechanism shown below for chlorination Chlorine Reaction Alkene Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. draw the structure of the product formed when a given alkene undergoes an addition. Chlorine Reaction Alkene.
From orgosolver.com
OrgoSolver Chlorine Reaction Alkene The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. These reactions are usually spontaneous. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens. Chlorine Reaction Alkene.
From www.coursehero.com
[Solved] Name the alkene that would react with chlorine to form the Chlorine Reaction Alkene write the equation for the reaction of chlorine or bromine with a given alkene. These reactions are usually spontaneous. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. Identify the conditions under which an. Chlorine Reaction Alkene.
From www.scribd.com
Alkene Reactions and Mechanisms Chlorination of Cyclohexene and Chlorine Reaction Alkene figure 8.5a addition reaction: Identify the conditions under which an addition reaction occurs between an alkene. draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. Addition of bromine and chlorine to alkenes. Chlorine Reaction Alkene.
From ar.inspiredpencil.com
Electrophilic Aromatic Substitution Mechanism Chlorination Chlorine Reaction Alkene in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to. draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. figure 8.5a addition reaction: Chlorination the reaction between c=c double bond and bromine (br 2 ) can. Chlorine Reaction Alkene.
From www.masterorganicchemistry.com
Alkyne Reaction Patterns The Carbocation Pathway — Master Organic Chlorine Reaction Alkene Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. These reactions are usually spontaneous. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. figure 8.5a addition reaction: write the equation for the reaction of chlorine or bromine. Chlorine Reaction Alkene.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Chlorine Reaction Alkene figure 8.5a addition reaction: The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. Identify the conditions under which an addition reaction occurs between an alkene. 10.4 reactions of alkenes: write the equation for the reaction of chlorine or bromine with a given alkene. in the presence of. Chlorine Reaction Alkene.
From solvedlib.com
Chlorine and bromine react in the dark with alkenes. … SolvedLib Chlorine Reaction Alkene When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. These reactions are usually spontaneous. Identify the conditions under which an addition reaction occurs between an alkene. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. figure 8.5a addition. Chlorine Reaction Alkene.
From www.organicchemistrytutor.com
Reactions of Alkenes — Organic Chemistry Tutor Chlorine Reaction Alkene write the equation for the reaction of chlorine or bromine with a given alkene. Identify the conditions under which an addition reaction occurs between an alkene. draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. These reactions are usually spontaneous. 10.4 reactions of alkenes: When alkenes (. Chlorine Reaction Alkene.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Chlorine Reaction Alkene These reactions are usually spontaneous. figure 8.5a addition reaction: chlorine, bromine or iodine can be added to an alkene. Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl.. Chlorine Reaction Alkene.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Chlorine Reaction Alkene When alkenes ( also known as olefins) are treated with bromine (br 2) or chlorine (cl. The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as cl2 or. 10.4 reactions of alkenes: figure 8.5a addition reaction: in the presence of aprotic solvent, the product is a vicinal dihalide, as shown here. Chlorine Reaction Alkene.
From www.chemistryscl.com
Benzene and Chlorine Reaction C6H6 + Cl2, Mechanism Chlorine Reaction Alkene 10.4 reactions of alkenes: Addition of bromine and chlorine to alkenes an addition reaction also easily occurs between halogens (br 2 and cl 2 ) and alkenes. Chlorination the reaction between c=c double bond and bromine (br 2 ) can be used as a test for. draw the structure of the product formed when a given alkene undergoes. Chlorine Reaction Alkene.