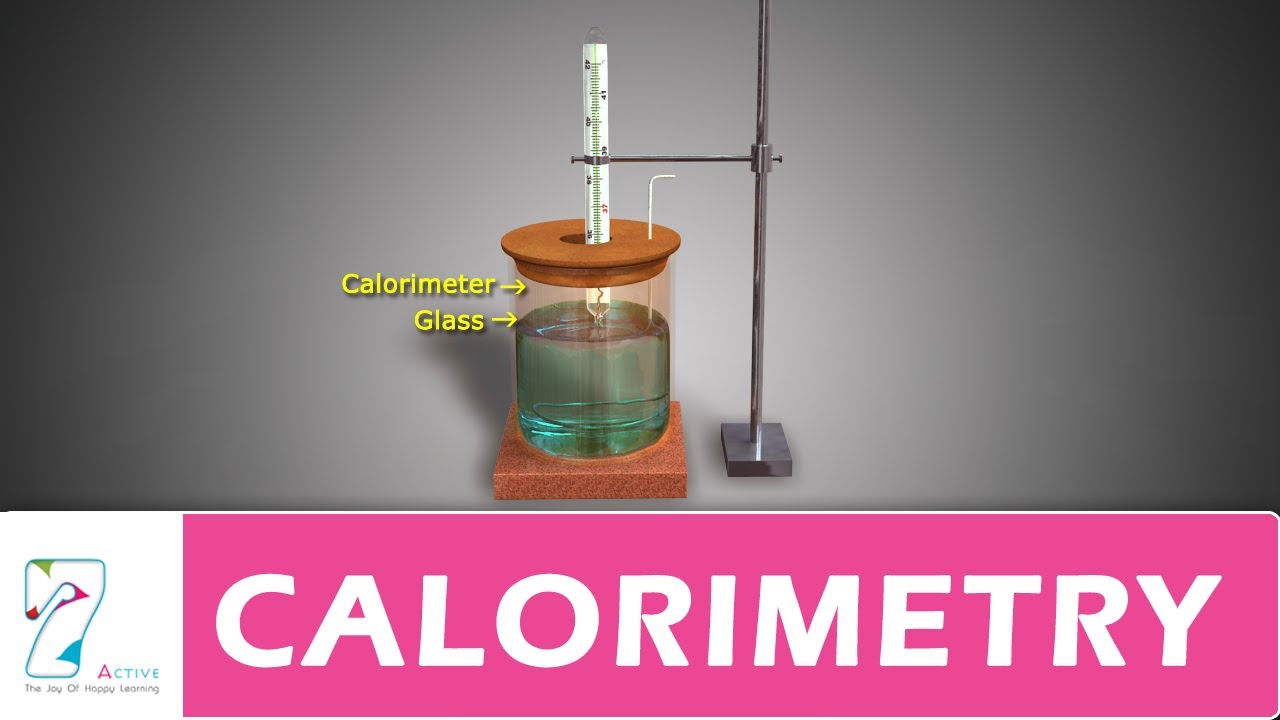
Why Is Calorimeter So Important . in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. Calorimeters play a crucial role in understanding the thermodynamics of a. To do so, the heat is exchanged with a. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. calorimetry is used to measure amounts of heat transferred to or from a substance. importance of calorimeters in chemistry. To do so, the heat is exchanged with a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is used to measure amounts of heat transferred to or from a substance. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings.
from chiangmaiplaces.net
calorimetry is used to measure amounts of heat transferred to or from a substance. importance of calorimeters in chemistry. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Calorimeters play a crucial role in understanding the thermodynamics of a. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. To do so, the heat is exchanged with a. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of.
How Do You Do A Calorimeter Laboratory? Trust The Answer
Why Is Calorimeter So Important calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. importance of calorimeters in chemistry. To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. Calorimeters play a crucial role in understanding the thermodynamics of a. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. To do so, the heat is exchanged with a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat.
From saylordotorg.github.io
Calorimetry Why Is Calorimeter So Important calorimetry is used to measure amounts of heat transferred to or from a substance. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. importance of calorimeters in chemistry. a calorimeter. Why Is Calorimeter So Important.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Why Is Calorimeter So Important similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. Calorimeters play a crucial role in understanding the thermodynamics of a. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. calorimetry is used to measure amounts of heat transferred to or from a. Why Is Calorimeter So Important.
From studylib.net
Calorimetry Why Is Calorimeter So Important calorimetry is used to measure amounts of heat transferred to or from a substance. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. calorimeters. Why Is Calorimeter So Important.
From www.youtube.com
050 Calorimetry YouTube Why Is Calorimeter So Important in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred. Why Is Calorimeter So Important.
From www.science-revision.co.uk
Calorimetry Why Is Calorimeter So Important calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. To do so, the heat is exchanged with a. similar to its name, constant volume calorimetry aims to maintain a constant volume. Why Is Calorimeter So Important.
From www.thoughtco.com
Calorimeter Definition in Chemistry Why Is Calorimeter So Important similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeters are designed to minimize. Why Is Calorimeter So Important.
From www.jove.com
Thermochemistry Constant Volume Calorimetry JoVE Book Why Is Calorimeter So Important To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. calorimeters are designed to minimize energy exchange between the. Why Is Calorimeter So Important.
From www.numerade.com
SOLVED Why is it important to determine the heat capacity of the Why Is Calorimeter So Important calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a.. Why Is Calorimeter So Important.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Why Is Calorimeter So Important similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well. Why Is Calorimeter So Important.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Why Is Calorimeter So Important calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. Calorimeters play a crucial role in understanding the thermodynamics of a. To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. a calorimeter is a device used. Why Is Calorimeter So Important.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Why Is Calorimeter So Important in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters play a crucial role in understanding the thermodynamics of a. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of. Why Is Calorimeter So Important.
From www.faviocoffee.com
Thermochemistry Enthalpy and Coffee Cup Calorimeter. Favio Coffee Why Is Calorimeter So Important calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. importance of calorimeters in chemistry. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat. Why Is Calorimeter So Important.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report Why Is Calorimeter So Important To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is. Why Is Calorimeter So Important.
From www.linstitute.net
AQA A Level Biology复习笔记5.3.3 Biomass翰林国际教育 Why Is Calorimeter So Important calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. importance of calorimeters in chemistry. To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure'). Why Is Calorimeter So Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimeter So Important calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. importance of calorimeters in chemistry. To do so, the heat is exchanged with a. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. in chemistry and thermodynamics, calorimetry (from latin calor. Why Is Calorimeter So Important.
From www.youtube.com
3 problems of specific heat & calorimeter (1st year secondary second Why Is Calorimeter So Important calorimetry is used to measure amounts of heat transferred to or from a substance. importance of calorimeters in chemistry. Calorimeters play a crucial role in understanding the thermodynamics of a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimeters are designed to minimize energy exchange. Why Is Calorimeter So Important.
From www.science-revision.co.uk
Calorimetry Why Is Calorimeter So Important calorimetry is used to measure amounts of heat transferred to or from a substance. importance of calorimeters in chemistry. Calorimeters play a crucial role in understanding the thermodynamics of a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. similar to its name, constant volume calorimetry. Why Is Calorimeter So Important.
From glossary.periodni.com
Download calorimeter.png image from Why Is Calorimeter So Important Calorimeters play a crucial role in understanding the thermodynamics of a. importance of calorimeters in chemistry. To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. a calorimeter is a device used for calorimetry, or the process of measuring the heat of. Why Is Calorimeter So Important.
From www.youtube.com
09.05 Calorimetry YouTube Why Is Calorimeter So Important calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Calorimeters play a crucial role in understanding the thermodynamics of a. To do so, the heat is exchanged with a. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. similar to its. Why Is Calorimeter So Important.
From socratic.org
A student conducting a calorimetry investigation determines a negative Why Is Calorimeter So Important To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. similar to its name, constant volume calorimetry aims to maintain a. Why Is Calorimeter So Important.
From chiangmaiplaces.net
How Do You Do A Calorimeter Laboratory? Trust The Answer Why Is Calorimeter So Important Calorimeters play a crucial role in understanding the thermodynamics of a. calorimetry is used to measure amounts of heat transferred to or from a substance. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. calorimetry is used to measure amounts of heat transferred to or from a substance.. Why Is Calorimeter So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Why Is Calorimeter So Important calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. calorimetry is used to measure amounts of heat transferred to or from a substance. importance of calorimeters. Why Is Calorimeter So Important.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Why Is Calorimeter So Important importance of calorimeters in chemistry. To do so, the heat is exchanged with a. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. calorimetry. Why Is Calorimeter So Important.
From www.youtube.com
Principle of Calorimetry YouTube Why Is Calorimeter So Important calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings.. Why Is Calorimeter So Important.
From manueldesnhray.blogspot.com
Identify What a Coffee Cup Calorimeter Measures. Why Is Calorimeter So Important importance of calorimeters in chemistry. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. To do so, the heat is exchanged with a.. Why Is Calorimeter So Important.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Why Is Calorimeter So Important To do so, the heat is exchanged with a. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is. Why Is Calorimeter So Important.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Why Is Calorimeter So Important in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a.. Why Is Calorimeter So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6505280 Why Is Calorimeter So Important a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. To do. Why Is Calorimeter So Important.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Why Is Calorimeter So Important calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. importance of calorimeters in chemistry. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. calorimetry is used to measure amounts of heat transferred to or from a substance.. Why Is Calorimeter So Important.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Why Is Calorimeter So Important importance of calorimeters in chemistry. To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is used to measure amounts of. Why Is Calorimeter So Important.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Why Is Calorimeter So Important Calorimeters play a crucial role in understanding the thermodynamics of a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. To do so, the heat is exchanged with a. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. To. Why Is Calorimeter So Important.
From users.highland.edu
Calorimetry Why Is Calorimeter So Important Calorimeters play a crucial role in understanding the thermodynamics of a. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used for calorimetry, or the process of measuring. Why Is Calorimeter So Important.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable Why Is Calorimeter So Important calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. To do so, the heat is exchanged with a. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. calorimetry is used. Why Is Calorimeter So Important.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Why Is Calorimeter So Important To do so, the heat is exchanged with a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat', and greek μέτρον (metron) 'measure') is the. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. calorimetry is used to measure amounts. Why Is Calorimeter So Important.
From www.numerade.com
SOLVED Why is it important to determine the heat capacity of the Why Is Calorimeter So Important To do so, the heat is exchanged with a. similar to its name, constant volume calorimetry aims to maintain a constant volume and resist large amounts of. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. importance of calorimeters in chemistry. calorimeter,. Why Is Calorimeter So Important.