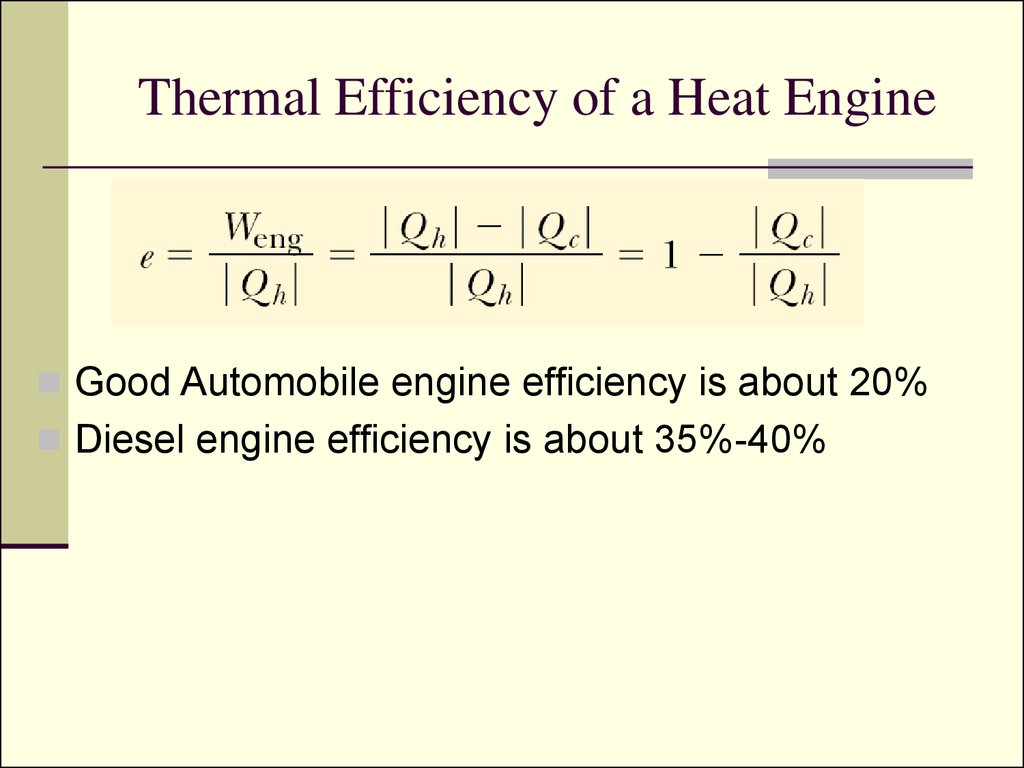
Using An Engine Of 30 Thermal Efficiency . a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. explain how dissipative processes affect the ideal carnot engine. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. It is impossible for heat engines to achieve 100% thermal efficiency. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that passively captures. We know from the second law of thermodynamics that a heat engine cannot be 100%. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. heat engines often operate at around 30% to 50% efficiency, due to practical limitations.
from workshoprepairarolla.z13.web.core.windows.net
achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. It is impossible for heat engines to achieve 100% thermal efficiency. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. We know from the second law of thermodynamics that a heat engine cannot be 100%. explain how dissipative processes affect the ideal carnot engine. the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that passively captures.
Formula For Efficiency Of Heat Engine
Using An Engine Of 30 Thermal Efficiency We know from the second law of thermodynamics that a heat engine cannot be 100%. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. It is impossible for heat engines to achieve 100% thermal efficiency. explain how dissipative processes affect the ideal carnot engine. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. We know from the second law of thermodynamics that a heat engine cannot be 100%. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that passively captures.
From www.youtube.com
Diesel Cycle Thermal Efficiency YouTube Using An Engine Of 30 Thermal Efficiency It is impossible for heat engines to achieve 100% thermal efficiency. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. explain how dissipative processes affect the ideal carnot engine. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. We. Using An Engine Of 30 Thermal Efficiency.
From thechemistrynotes.com
Heat Engine Principle, Parts, Importance The Chemistry Notes Using An Engine Of 30 Thermal Efficiency heat engines often operate at around 30% to 50% efficiency, due to practical limitations. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. It is impossible for. Using An Engine Of 30 Thermal Efficiency.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID315568 Using An Engine Of 30 Thermal Efficiency in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. We know. Using An Engine Of 30 Thermal Efficiency.
From repairmachinepartizanugv.z22.web.core.windows.net
Thermal Efficiency Of Diesel Engine Formula Using An Engine Of 30 Thermal Efficiency heat engines often operate at around 30% to 50% efficiency, due to practical limitations. explain how dissipative processes affect the ideal carnot engine. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that. Using An Engine Of 30 Thermal Efficiency.
From fixengineextroverts101.z21.web.core.windows.net
Thermal Efficiency Of Diesel Engine Formula Using An Engine Of 30 Thermal Efficiency in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. We know from the second law of thermodynamics that a heat engine cannot be 100%. explain how dissipative processes affect the. Using An Engine Of 30 Thermal Efficiency.
From www.researchgate.net
Brake thermal efficiency vs. engine speed. Download Scientific Diagram Using An Engine Of 30 Thermal Efficiency We know from the second law of thermodynamics that a heat engine cannot be 100%. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. achieving this goal would. Using An Engine Of 30 Thermal Efficiency.
From mechanicbibellogmi.z14.web.core.windows.net
Thermal Efficiency Of Diesel And Lng Engine Using An Engine Of 30 Thermal Efficiency explain how dissipative processes affect the ideal carnot engine. We know from the second law of thermodynamics that a heat engine cannot be 100%. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. a way to quantify how efficiently a machine runs is through. Using An Engine Of 30 Thermal Efficiency.
From www.omnicalculator.com
Thermal Efficiency Calculator Using An Engine Of 30 Thermal Efficiency heat engines often operate at around 30% to 50% efficiency, due to practical limitations. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. the heat engine is a thermophotovoltaic (tpv) cell, similar. Using An Engine Of 30 Thermal Efficiency.
From repairmachinetshileshepl.z4.web.core.windows.net
Heat Engine And Efficiency Using An Engine Of 30 Thermal Efficiency heat engines often operate at around 30% to 50% efficiency, due to practical limitations. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. write down the expression. Using An Engine Of 30 Thermal Efficiency.
From fixmachinebronchos101.z21.web.core.windows.net
Thermal Efficiency Of Diesel Engine Using An Engine Of 30 Thermal Efficiency explain how dissipative processes affect the ideal carnot engine. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that passively captures. a way to quantify how efficiently a. Using An Engine Of 30 Thermal Efficiency.
From www.slideserve.com
PPT Energy Systems for Surface Transport2 PowerPoint Presentation, free download ID163207 Using An Engine Of 30 Thermal Efficiency explain how dissipative processes affect the ideal carnot engine. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. It is impossible for heat engines to achieve 100% thermal efficiency. . Using An Engine Of 30 Thermal Efficiency.
From byjus.com
What is the formula for thermal efficiency? Using An Engine Of 30 Thermal Efficiency It is impossible for heat engines to achieve 100% thermal efficiency. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. We know from the second law of thermodynamics that. Using An Engine Of 30 Thermal Efficiency.
From www.sennoparts.com
Exploring the Thermal Efficiency of Diesel Engines Using An Engine Of 30 Thermal Efficiency a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. We know from the second law of thermodynamics that a. Using An Engine Of 30 Thermal Efficiency.
From workshoprepairarolla.z13.web.core.windows.net
Formula For Efficiency Of Heat Engine Using An Engine Of 30 Thermal Efficiency heat engines often operate at around 30% to 50% efficiency, due to practical limitations. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. It is impossible for heat engines to achieve 100% thermal efficiency. in the carnot engine, which is the most efficient conceivable engine for given source and sink. Using An Engine Of 30 Thermal Efficiency.
From videouroki.net
Thermal engines. Principle of operation of thermal engines. Internal combustion engine Using An Engine Of 30 Thermal Efficiency write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. We know from the second law of thermodynamics that a heat engine cannot be 100%. the heat engine is a thermophotovoltaic (tpv) cell, similar to a. Using An Engine Of 30 Thermal Efficiency.
From fixmachinemistiming.z22.web.core.windows.net
Define Thermal Efficiency Of Heat Engine Using An Engine Of 30 Thermal Efficiency We know from the second law of thermodynamics that a heat engine cannot be 100%. the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that passively captures. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. write down the expression for thermal efficiency of heat engine. Using An Engine Of 30 Thermal Efficiency.
From fixmachinebronchos101.z21.web.core.windows.net
Efficiency Of Heat Engine Formula Using An Engine Of 30 Thermal Efficiency explain how dissipative processes affect the ideal carnot engine. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. It is impossible for heat engines to achieve 100% thermal efficiency. We know from the second law of thermodynamics that a heat engine cannot be 100%. write down the expression for thermal. Using An Engine Of 30 Thermal Efficiency.
From journals.sagepub.com
Technical research on improving engine thermal efficiency Hongyu Mu, Yinyan Wang, Chuanlei Using An Engine Of 30 Thermal Efficiency achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. explain how dissipative processes affect the ideal carnot engine. It is impossible for heat engines to achieve 100% thermal efficiency. in the carnot engine, which is the most efficient conceivable engine for given source and. Using An Engine Of 30 Thermal Efficiency.
From coratololfefixmachine.z13.web.core.windows.net
Thermal Efficiency Of Diesel Engine Using An Engine Of 30 Thermal Efficiency the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that passively captures. It is impossible for heat engines to achieve 100% thermal efficiency. explain how dissipative processes affect the ideal carnot engine. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. achieving this goal would. Using An Engine Of 30 Thermal Efficiency.
From www.slideserve.com
PPT Engine Parameters PowerPoint Presentation, free download ID3484043 Using An Engine Of 30 Thermal Efficiency a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. We know from the second law of thermodynamics that a heat engine cannot be 100%. explain how dissipative processes affect the ideal carnot engine. It is impossible for heat engines to achieve 100% thermal efficiency. the heat engine is a thermophotovoltaic. Using An Engine Of 30 Thermal Efficiency.
From machinepovlakteeeh.z21.web.core.windows.net
Thermal Efficiency Of Diesel Engine Formula Using An Engine Of 30 Thermal Efficiency heat engines often operate at around 30% to 50% efficiency, due to practical limitations. It is impossible for heat engines to achieve 100% thermal efficiency. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. explain how dissipative processes affect the ideal carnot engine. . Using An Engine Of 30 Thermal Efficiency.
From heatenginekiechiko.blogspot.com
Heat Engine Thermal Efficiency Of Heat Engine Using An Engine Of 30 Thermal Efficiency write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that passively captures.. Using An Engine Of 30 Thermal Efficiency.
From www.slideserve.com
PPT THERMODYNAMIC an introduction PowerPoint Presentation, free download ID2817183 Using An Engine Of 30 Thermal Efficiency the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that passively captures. It is impossible for heat engines to achieve 100% thermal efficiency. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. We know from the second law of thermodynamics that a heat engine cannot be 100%.. Using An Engine Of 30 Thermal Efficiency.
From www.slideserve.com
PPT Energy in thermal processes PowerPoint Presentation, free download ID3480002 Using An Engine Of 30 Thermal Efficiency We know from the second law of thermodynamics that a heat engine cannot be 100%. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. the heat engine. Using An Engine Of 30 Thermal Efficiency.
From www.youtube.com
Thermodynamics Second Law Introduction, Thermal Efficiency, Heat Engines YouTube Using An Engine Of 30 Thermal Efficiency in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. explain how dissipative. Using An Engine Of 30 Thermal Efficiency.
From www.researchgate.net
Variation of brake thermal efficiency with engine speeds for fuel... Download Scientific Diagram Using An Engine Of 30 Thermal Efficiency It is impossible for heat engines to achieve 100% thermal efficiency. We know from the second law of thermodynamics that a heat engine cannot be 100%. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. the. Using An Engine Of 30 Thermal Efficiency.
From www.slideserve.com
PPT Chapter 9 PowerPoint Presentation, free download ID5249149 Using An Engine Of 30 Thermal Efficiency It is impossible for heat engines to achieve 100% thermal efficiency. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. the heat engine is a thermophotovoltaic (tpv) cell, similar to a solar panel’s photovoltaic cells, that passively captures. write down the expression for thermal. Using An Engine Of 30 Thermal Efficiency.
From mechanicdbminimizes.z14.web.core.windows.net
Thermal Efficiency Of Carnot Engine Formula Using An Engine Of 30 Thermal Efficiency in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. We know from the second law of thermodynamics that a heat engine cannot be. Using An Engine Of 30 Thermal Efficiency.
From repairfixrepository.z13.web.core.windows.net
Heat Engine Equations Using An Engine Of 30 Thermal Efficiency a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. the. Using An Engine Of 30 Thermal Efficiency.
From repairmachinetshileshepl.z4.web.core.windows.net
Maximum Theoretical Efficiency Of Heat Engine Using An Engine Of 30 Thermal Efficiency a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. We know. Using An Engine Of 30 Thermal Efficiency.
From mechanictalonerag1.z13.web.core.windows.net
Thermal Efficiency Of Heat Engine Formula Using An Engine Of 30 Thermal Efficiency We know from the second law of thermodynamics that a heat engine cannot be 100%. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. a way to quantify how efficiently a machine runs is through a quantity called thermal efficiency. explain how dissipative processes affect the ideal carnot engine.. Using An Engine Of 30 Thermal Efficiency.
From garagefixstrafes.z22.web.core.windows.net
Thermal Efficiency Of Jet Engine Formula Using An Engine Of 30 Thermal Efficiency We know from the second law of thermodynamics that a heat engine cannot be 100%. in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. a way to quantify how efficiently. Using An Engine Of 30 Thermal Efficiency.
From www.researchgate.net
Thermal efficiency vs engine speed Download Scientific Diagram Using An Engine Of 30 Thermal Efficiency in the carnot engine, which is the most efficient conceivable engine for given source and sink temperature, the efficiency is \[ \eta=\frac{t_{2}. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it has a thermal efficiency. We know from the second law of thermodynamics that a heat engine cannot be. Using An Engine Of 30 Thermal Efficiency.
From www.researchgate.net
Engine Load versus Thermal Efficiency Download Scientific Diagram Using An Engine Of 30 Thermal Efficiency heat engines often operate at around 30% to 50% efficiency, due to practical limitations. write down the expression for thermal efficiency of heat engine and coefficient of performance (cop) of the. We know from the second law of thermodynamics that a heat engine cannot be 100%. explain how dissipative processes affect the ideal carnot engine. achieving. Using An Engine Of 30 Thermal Efficiency.
From www.slideserve.com
PPT Engine Terminology PowerPoint Presentation, free download ID3018021 Using An Engine Of 30 Thermal Efficiency It is impossible for heat engines to achieve 100% thermal efficiency. heat engines often operate at around 30% to 50% efficiency, due to practical limitations. We know from the second law of thermodynamics that a heat engine cannot be 100%. achieving this goal would mean the creation of a “perfectly efficient engine,” and we would say that it. Using An Engine Of 30 Thermal Efficiency.