Kpa Gas Constant . Learn how r relates to the boltzmann constant, the specific gas. the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. r is the gas constant used in the ideal gas law and nernst equation. \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. for an ideal gas, the product pv (p: the ideal gas constant is calculated to be \(8.314 \: use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant.
from www.chegg.com
for an ideal gas, the product pv (p: learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. r is the gas constant used in the ideal gas law and nernst equation. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). Learn how r relates to the boltzmann constant, the specific gas. use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. the ideal gas constant is calculated to be \(8.314 \:
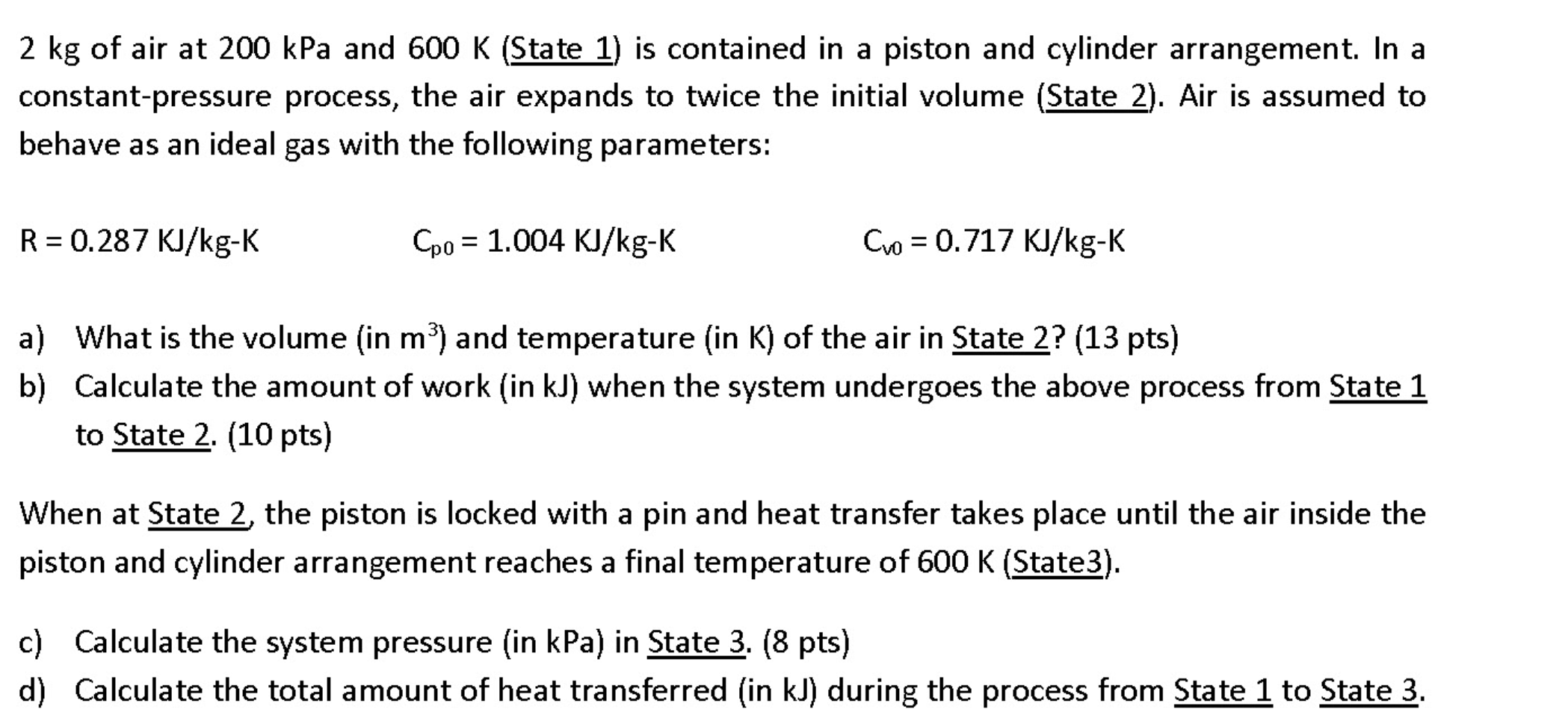
Solved 2 kg of air at 200 kPa and 600 K (State 1) is
Kpa Gas Constant learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. for an ideal gas, the product pv (p: learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. r is the gas constant used in the ideal gas law and nernst equation. \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. the ideal gas constant is calculated to be \(8.314 \: Learn how r relates to the boltzmann constant, the specific gas. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into.
From www.toppr.com
Gas Constant Definition, Formula, Ideal Gas and Examples Kpa Gas Constant \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. the ideal gas constant is calculated to be \(8.314 \: learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. . Kpa Gas Constant.
From slideplayer.com
The Gas Laws Chemistry Dr. May. ppt download Kpa Gas Constant for an ideal gas, the product pv (p: Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). the ideal gas constant is calculated to be \(8.314 \: the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. use the ideal gas. Kpa Gas Constant.
From www.chegg.com
Solved Boyle's Law states that when a sample of gas is Kpa Gas Constant learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. Learn how r relates to the boltzmann constant, the specific gas. use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. for an ideal gas, the. Kpa Gas Constant.
From www.numerade.com
SOLVED An ideal gas mixture consists of 3 kg of nitrogen and 5 kg of Kpa Gas Constant for an ideal gas, the product pv (p: \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. r is the gas constant used in the ideal gas law and nernst equation. the ideal gas constant is. Kpa Gas Constant.
From www.numerade.com
SOLVED Two dm3 of N2 at 0oC and 500 kPa pressure are expanded Kpa Gas Constant use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Learn how r relates to the boltzmann constant, the specific gas. learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. Volume) is a constant if the. Kpa Gas Constant.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation ID1278113 Kpa Gas Constant use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. the ideal gas constant is calculated to be \(8.314 \: learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. Volume) is a constant if the. Kpa Gas Constant.
From www.chegg.com
Solved Gas Constant 8.314 kJ/(kmolK) 8.314 kPam3/(kmolK) Kpa Gas Constant use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. Learn how r relates to the boltzmann constant, the specific gas. for an. Kpa Gas Constant.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation ID5758540 Kpa Gas Constant the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. r is the gas constant used in the ideal gas law and nernst equation. Learn how r relates to the boltzmann constant, the specific gas. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s. Kpa Gas Constant.
From www.slideserve.com
PPT 1. The volume of a gas at 99.0 kPa is 300.0 mL. If the pressure Kpa Gas Constant \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. for an ideal gas, the product pv (p: use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Learn how r relates to the boltzmann constant, the specific gas. Volume) is a constant if the. Kpa Gas Constant.
From www.chegg.com
Solved Neon is compressed from 100 kPa and 21°C to 500 kPa Kpa Gas Constant the ideal gas constant is calculated to be \(8.314 \: learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. Learn how r relates to the boltzmann constant, the specific gas. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). for an ideal gas, the. Kpa Gas Constant.
From www.slideserve.com
PPT THE PROPERTIES OF GASES PowerPoint Presentation, free download Kpa Gas Constant Learn how r relates to the boltzmann constant, the specific gas. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. the ideal gas constant is calculated to be \(8.314 \: \text{j/k} \cdot \text{mol}\) when the pressure. Kpa Gas Constant.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Kpa Gas Constant learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. Learn how r relates to the boltzmann constant, the specific gas. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). the ideal gas constant is calculated to. Kpa Gas Constant.
From slideplayer.com
Chapter 12 Notes, Part II Ideal Gas Law ppt download Kpa Gas Constant use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. for an ideal gas, the product pv (p: Learn how r relates to the boltzmann constant, the specific gas. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). \text{j/k}. Kpa Gas Constant.
From www.slideserve.com
PPT Chapter 5 Gases and the Molecular Theory PowerPoint Kpa Gas Constant r is the gas constant used in the ideal gas law and nernst equation. the ideal gas constant is calculated to be \(8.314 \: the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. learn about the assumptions and applications of the ideal gas law, which. Kpa Gas Constant.
From www.yakimankagbu.ru
Kpa Unit Store Sale www.yakimankagbu.ru Kpa Gas Constant the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. r is the gas constant used in the ideal gas law and nernst equation. the ideal gas constant is calculated to be \(8.314 \: learn about the. Kpa Gas Constant.
From www.slideserve.com
PPT Thermodynamic Properties PowerPoint Presentation, free download Kpa Gas Constant \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. the ideal gas constant is calculated to be \(8.314 \: Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). r is the gas constant used in the ideal gas law and nernst equation. the empirical relationships among the volume, the temperature, the pressure,. Kpa Gas Constant.
From www.chegg.com
Values For The Gas Constant 14.7 Psia 101.3 KPa 21... Kpa Gas Constant learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. for an ideal gas, the product pv (p: the ideal gas constant is calculated to be \(8.314 \: Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). use the ideal gas equation to solve. Kpa Gas Constant.
From www.youtube.com
An ideal gas expands while the pressure is kept constant. During the Kpa Gas Constant for an ideal gas, the product pv (p: the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. Learn how r relates to the boltzmann constant, the specific gas. . Kpa Gas Constant.
From www.chegg.com
Solved 1. Problem 1 Air (ideal gas) at pressure 100 kPa and Kpa Gas Constant use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). for an. Kpa Gas Constant.
From www.slideserve.com
PPT Gas Relationships PowerPoint Presentation, free download ID2707799 Kpa Gas Constant the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. Learn how r relates to the boltzmann constant, the specific gas. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). use the ideal gas equation to solve a problem when the amount of. Kpa Gas Constant.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID3344204 Kpa Gas Constant r is the gas constant used in the ideal gas law and nernst equation. the ideal gas constant is calculated to be \(8.314 \: use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. \text{j/k} \cdot \text{mol}\) when the pressure is in kpa.. Kpa Gas Constant.
From mmerevise.co.uk
The Ideal Gas Equation MME Kpa Gas Constant the ideal gas constant is calculated to be \(8.314 \: learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Volume) is a constant if the. Kpa Gas Constant.
From www.chegg.com
Solved Create A Function To Convert R (ideal Gas Constant... Kpa Gas Constant the ideal gas constant is calculated to be \(8.314 \: the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. Learn how r relates to the boltzmann constant, the specific gas. use the ideal gas equation to solve a problem when the amount of gas is given. Kpa Gas Constant.
From brainly.ph
At 32 degrees and 205 kpa gage the specific weight of a certain gas was Kpa Gas Constant r is the gas constant used in the ideal gas law and nernst equation. learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. for an ideal gas, the. Kpa Gas Constant.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 Kpa Gas Constant the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. the ideal gas constant is calculated to be \(8.314 \: Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). learn about the assumptions and applications of the ideal gas law, which relates. Kpa Gas Constant.
From www.slideserve.com
PPT General Chemistry PowerPoint Presentation, free download ID5940887 Kpa Gas Constant Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). Learn how r relates to the boltzmann constant, the specific gas. r is the gas constant used in the ideal gas law and nernst equation. for an ideal gas, the product pv (p: the ideal gas constant is calculated to be \(8.314 \:. Kpa Gas Constant.
From www.chegg.com
Solved 1. An ideal gas at atmospheric pressure (101.3 kPa) Kpa Gas Constant Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Learn how r relates to the boltzmann constant, the specific gas. r is the gas constant used in the ideal. Kpa Gas Constant.
From cewzpojc.blob.core.windows.net
Gas Law Equation Calculator at Sandra Kell blog Kpa Gas Constant the ideal gas constant is calculated to be \(8.314 \: for an ideal gas, the product pv (p: learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. r is the gas constant used in the ideal gas law and nernst equation. Volume) is a constant if the gas. Kpa Gas Constant.
From www.slideserve.com
PPT Chapter 10 Gases PowerPoint Presentation, free download ID5339732 Kpa Gas Constant r is the gas constant used in the ideal gas law and nernst equation. for an ideal gas, the product pv (p: Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). Learn how r relates to the boltzmann constant, the specific gas. the ideal gas constant is calculated to be \(8.314 \:. Kpa Gas Constant.
From www.numerade.com
The Henry's Law constant for CO2 in water at 37 '€ is 3.30 x 104 mol L Kpa Gas Constant Learn how r relates to the boltzmann constant, the specific gas. learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. r is the gas constant used in the ideal gas law and nernst equation. use the ideal gas equation to solve a problem when the amount of gas is. Kpa Gas Constant.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4199554 Kpa Gas Constant learn about the assumptions and applications of the ideal gas law, which relates pressure, volume, number of. \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. Learn how r relates to the boltzmann constant, the specific gas. . Kpa Gas Constant.
From www.thermopedia.com
GAS LAW CONSTANTS Kpa Gas Constant for an ideal gas, the product pv (p: the ideal gas constant is calculated to be \(8.314 \: \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. Learn how r relates to the boltzmann constant, the specific. Kpa Gas Constant.
From www.chegg.com
Solved A 20kg Mass Of Helium Is Maintained At 300 KPa An... Kpa Gas Constant Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). for an ideal gas, the product pv (p: the ideal gas constant is calculated to be \(8.314 \: r is the gas constant used in the ideal gas law and nernst equation. learn about the assumptions and applications of the ideal gas. Kpa Gas Constant.
From www.chegg.com
Solved 2 kg of air at 200 kPa and 600 K (State 1) is Kpa Gas Constant Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). the empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into. Learn how r relates to the boltzmann constant, the specific gas. the ideal gas constant is calculated to be \(8.314 \: use the. Kpa Gas Constant.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Kpa Gas Constant Learn how r relates to the boltzmann constant, the specific gas. Volume) is a constant if the gas is kept at isothermal conditions (boyle’s law). \text{j/k} \cdot \text{mol}\) when the pressure is in kpa. r is the gas constant used in the ideal gas law and nernst equation. learn about the assumptions and applications of the ideal gas. Kpa Gas Constant.