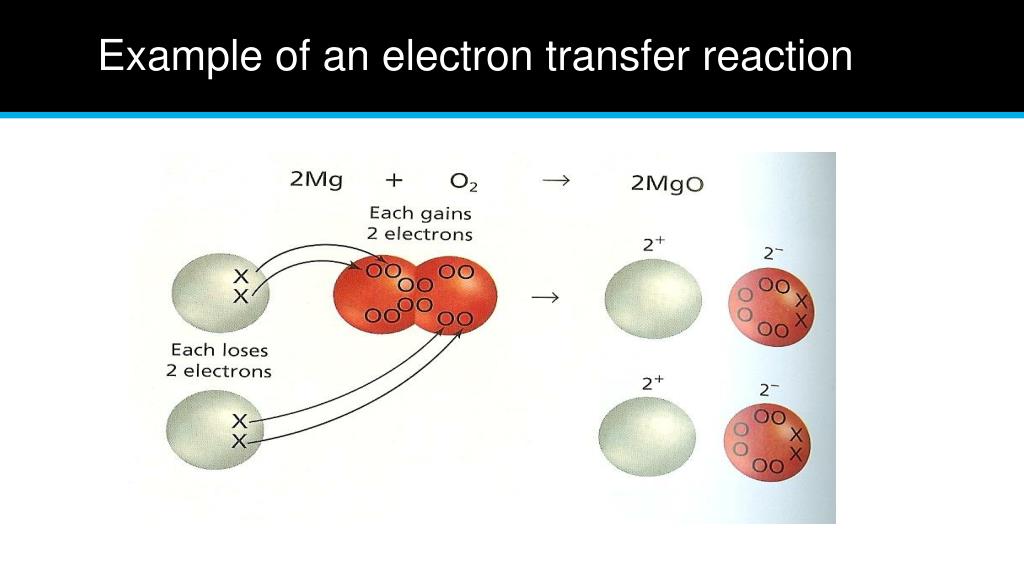
What Are Transfer Electron . Electron transfer is a process by which an electron moves from one atom or molecule to another. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. It is a key concept in. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond.
from www.slideserve.com
It is a key concept in. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is a process by which an electron moves from one atom or molecule to another. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the.
PPT Oxidation and Reduction PowerPoint Presentation, free download
What Are Transfer Electron The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. It is a key concept in. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the.
From studylib.net
Theory of ProtonCoupled Electron Transfer What Are Transfer Electron The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds. What Are Transfer Electron.
From www.slideserve.com
PPT Electron Transfer Reactions PowerPoint Presentation, free What Are Transfer Electron Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. It is a key concept in. Electron transfer is a process by which an electron moves from one atom or. What Are Transfer Electron.
From www.slideserve.com
PPT Photoinduced Electron Transfer in a DonorAcceptor Dyad What Are Transfer Electron The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. It is a key concept in. Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction. What Are Transfer Electron.
From www.slideserve.com
PPT Chemical Bonding Basic Concepts PowerPoint Presentation, free What Are Transfer Electron The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is a process by which an electron moves from one atom or molecule to another. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. Electron transfer reaction is a reaction in which a single electron. What Are Transfer Electron.
From www.slideserve.com
PPT Bonding Between Atoms PowerPoint Presentation, free download ID What Are Transfer Electron Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is a. What Are Transfer Electron.
From www.slideserve.com
PPT Chapters 1234 PowerPoint Presentation, free download ID1459282 What Are Transfer Electron Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. The attraction between oppositely charged ions is called an ionic bond,. What Are Transfer Electron.
From www.youtube.com
TYPES OF ELECTRON TRANSFER REACTIONS, Class 11 YouTube What Are Transfer Electron Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. Electron transfer reaction is a. What Are Transfer Electron.
From www.expii.com
Electron Transport Chain — Summary & Diagrams Expii What Are Transfer Electron Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. The strength of ionic bonding. What Are Transfer Electron.
From www.slideserve.com
PPT Electron Transfer Reactions PowerPoint Presentation, free What Are Transfer Electron The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. Electron transfer is a process by which an electron moves from one atom or molecule to another. Electron transfer reaction is a. What Are Transfer Electron.
From www.researchgate.net
Extracellular electron transfer. Means of electron transfer within a What Are Transfer Electron The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is a process by which an electron moves from one atom or molecule to another. It is a key concept in. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. Electron transfer reaction is a. What Are Transfer Electron.
From spmchemistry.blog.onlinetuition.com.my
Transfer of Electrons from One Point to Another SPM Chemistry What Are Transfer Electron Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. It is a key concept in. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation. What Are Transfer Electron.
From step1.medbullets.com
Electron Transport Chain Biochemistry Medbullets Step 1 What Are Transfer Electron The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. It is a key concept. What Are Transfer Electron.
From www.researchgate.net
Layerdependent electron transfer mechanism of MoS2 a, b Schematic What Are Transfer Electron It is a key concept in. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is a process by which an electron moves from one atom. What Are Transfer Electron.
From studylib.net
Chapter 20 Electron Transfer Reactions What Are Transfer Electron Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. It is a key concept in. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. Electron transfer reaction is a reaction in which. What Are Transfer Electron.
From www.slideserve.com
PPT Current and Potential Difference PowerPoint Presentation, free What Are Transfer Electron Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. Electron transfer is a process by which an electron moves from one atom or molecule to another. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. The attraction between. What Are Transfer Electron.
From ibiologia.com
Electron Transport Chain Introduction , Steps & Examples What Are Transfer Electron It is a key concept in. Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. The attraction of oppositely charged ions caused by electron transfer is called an ionic. What Are Transfer Electron.
From www.researchgate.net
5. The three known methods of electron transfer demonstrated by What Are Transfer Electron It is a key concept in. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. Electron transfer is the fundamental process in which electrons move from one atom or molecule to. What Are Transfer Electron.
From www.slideserve.com
PPT ELECTRON TRANSFER REACTIONS Some Basic Principles PowerPoint What Are Transfer Electron Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. It is a key. What Are Transfer Electron.
From www.youtube.com
Outer Sphere Electron Transfer Reactions. YouTube What Are Transfer Electron Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. It is a key concept. What Are Transfer Electron.
From spmchemistry.blog.onlinetuition.com.my
Transfer of Electrons from One Point to Another SPM Chemistry What Are Transfer Electron Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. It is a key concept in. Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction. What Are Transfer Electron.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download What Are Transfer Electron The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is a process by which an electron moves from one atom or molecule to another. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is the fundamental process in which electrons move. What Are Transfer Electron.
From physicsworld.com
Protoncoupled electron transfer in electrochemistry Physics World What Are Transfer Electron Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is a process by which an electron moves from one atom or molecule to another. It is a key concept in. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in. What Are Transfer Electron.
From www.vectorstock.com
Redox reaction with electron transfer Royalty Free Vector What Are Transfer Electron The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. It is a key concept in. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is the. What Are Transfer Electron.
From schematicdiagrampoukes.z13.web.core.windows.net
Electron Transfer Diagrams What Are Transfer Electron The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer is a process by which an electron moves from one atom or molecule to another. The strength of ionic bonding. What Are Transfer Electron.
From www.researchgate.net
Mechanisms involved in electron transfer (A) Indirect transfer via What Are Transfer Electron The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. It is a key concept in. Electron transfer is the. What Are Transfer Electron.
From www.slideserve.com
PPT Chapter 20 Principles of Chemical Reactivity Electron Transfer What Are Transfer Electron The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. It is a key concept in. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule. What Are Transfer Electron.
From www.chemtube3d.com
Inner Sphere Electron Transfer Mechanism What Are Transfer Electron Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is a process by which an electron moves from one atom or molecule to another. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. The attraction of oppositely charged ions caused by. What Are Transfer Electron.
From www.britannica.com
Electron transport chain biochemistry Britannica What Are Transfer Electron It is a key concept in. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. The strength of ionic bonding depends on the magnitude of the charges and. What Are Transfer Electron.
From microbenotes.com
Electron Transport Chain Steps, Products, Diagram What Are Transfer Electron The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. It is a key concept in. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction between oppositely. What Are Transfer Electron.
From opentextbc.ca
2.1 The Building Blocks of Molecules Concepts of Biology1st Canadian What Are Transfer Electron The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. The strength of ionic. What Are Transfer Electron.
From www.researchgate.net
Direct electron transfer mechanisms via membraneattached cytochrome What Are Transfer Electron It is a key concept in. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds. What Are Transfer Electron.
From www.slideserve.com
PPT Principle of the electron transport chain. PowerPoint What Are Transfer Electron The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. It is a key concept in. Electron transfer is the fundamental process in which electrons move from one atom or. What Are Transfer Electron.
From www.slideserve.com
PPT Electron Transfer Reactions PowerPoint Presentation, free What Are Transfer Electron The attraction of oppositely charged ions caused by electron transfer is called an ionic bond. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. Electron transfer reaction is a reaction in which a single electron is transferred from one molecule to another. It is a key. What Are Transfer Electron.
From www.slideserve.com
PPT Energy and Electron Transfer PowerPoint Presentation, free What Are Transfer Electron The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the. Electron transfer is a process by which an electron moves from one atom or molecule to another. Electron transfer reaction. What Are Transfer Electron.
From www.researchgate.net
Scheme 3 (a) The single electron transfer mechanism for the covalent What Are Transfer Electron It is a key concept in. Electron transfer is the fundamental process in which electrons move from one atom or molecule to another, resulting in the formation of ionic. Electron transfer is a process by which an electron moves from one atom or molecule to another. The attraction between oppositely charged ions is called an ionic bond, and it is. What Are Transfer Electron.