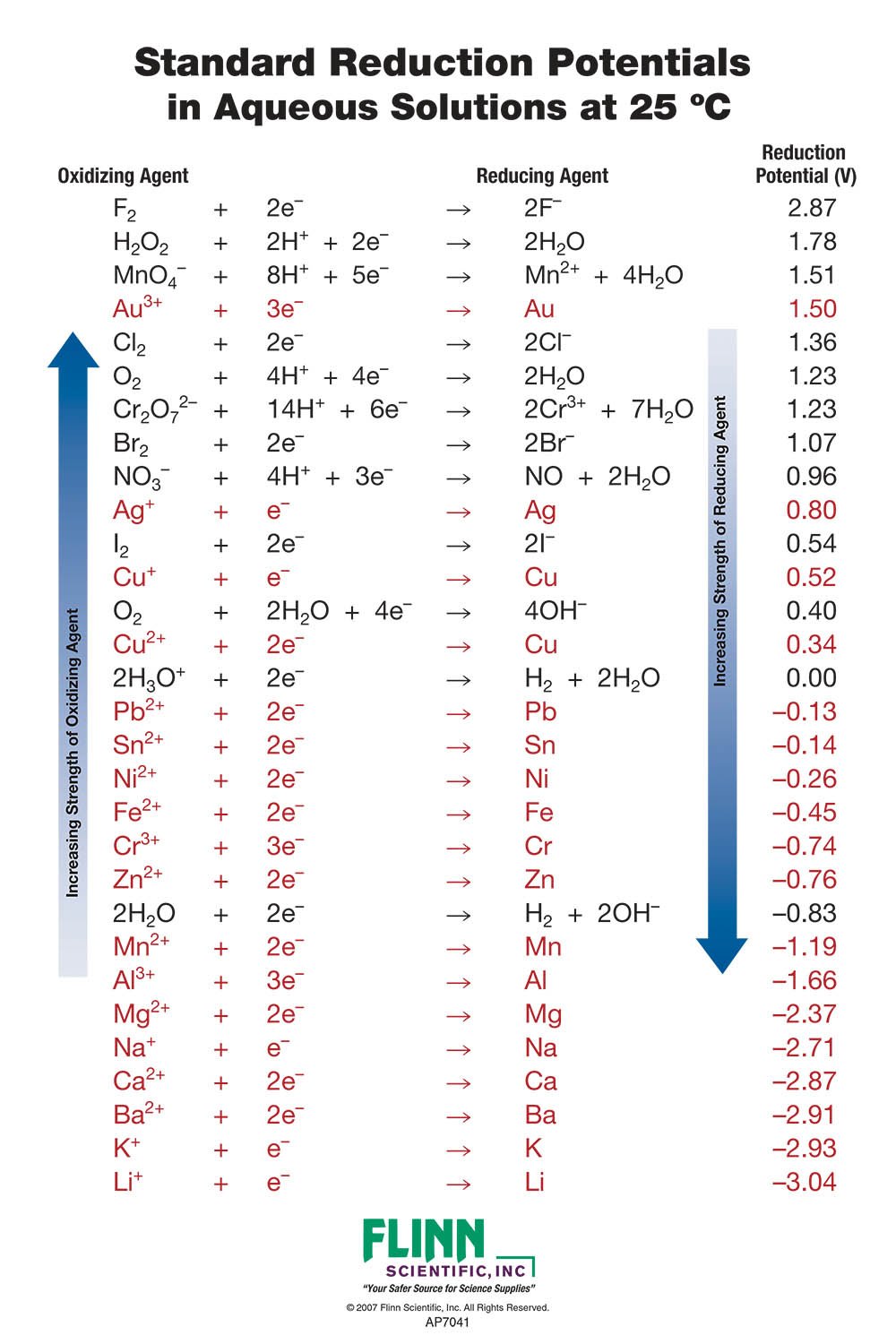
Standard Reduction Potential Values Metallic . standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Standard reduction potentials by element. standard reduction potential (e°): Standard reduction potentials by value. The value of the reduction under standard conditions (1 bar or 1 atm for gases; The following table provides eo and eo ´ values for. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. The following table provides eo for selected reduction. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 1 m for solutes) usually at 298.15 k;.
from www.flinnsci.ca
372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard reduction potentials by element. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. 1 m for solutes) usually at 298.15 k;. The following table provides eo for selected reduction. Standard reduction potentials by value. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. standard reduction potential (e°): The following table provides eo and eo ´ values for.
Standard Reduction Potential Charts for Chemistry
Standard Reduction Potential Values Metallic the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Standard reduction potentials by value. standard reduction potential (e°): in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. 1 m for solutes) usually at 298.15 k;. The following table provides eo and eo ´ values for. Standard reduction potentials by element. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The following table provides eo for selected reduction.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Values Metallic standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. standard reduction potential (e°): 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 1 m for solutes) usually at 298.15 k;. The value of the reduction under standard. Standard Reduction Potential Values Metallic.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Values Metallic Standard reduction potentials by element. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. standard reduction potential (e°): The following table provides. Standard Reduction Potential Values Metallic.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free Standard Reduction Potential Values Metallic the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. in this video, we're gonna take a look at an old concept of oxidation and. Standard Reduction Potential Values Metallic.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download Standard Reduction Potential Values Metallic in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by element. standard reduction potential (e°): The following table provides eo for selected reduction. the standard reduction potential is the tendency for. Standard Reduction Potential Values Metallic.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Values Metallic the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. standard reduction potential (e°): 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. in this video, we're gonna take a look at an. Standard Reduction Potential Values Metallic.
From www.sliderbase.com
Electrochemical Terminology Standard Reduction Potential Values Metallic Standard reduction potentials by element. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. 1 m for solutes) usually at 298.15 k;. standard reduction potential (e°): Standard reduction potentials by value. The following table provides eo for selected reduction. the standard reduction. Standard Reduction Potential Values Metallic.
From ch302.cm.utexas.edu
Electrochemistry_Reduction_Potentials Standard Reduction Potential Values Metallic The value of the reduction under standard conditions (1 bar or 1 atm for gases; in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. . Standard Reduction Potential Values Metallic.
From www.chegg.com
Solved Standard reduction potentials Use the table of Standard Reduction Potential Values Metallic The following table provides eo and eo ´ values for. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. 1 m for solutes) usually at 298.15 k;. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain. Standard Reduction Potential Values Metallic.
From www.researchgate.net
Standard reduction potential (V vs Ag/AgCl) of different elements in Standard Reduction Potential Values Metallic 1 m for solutes) usually at 298.15 k;. The following table provides eo for selected reduction. standard reduction potential (e°): the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. in this video, we're gonna take a look at an old concept of oxidation and. Standard Reduction Potential Values Metallic.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Values Metallic The value of the reduction under standard conditions (1 bar or 1 atm for gases; standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value. in this video, we're gonna take a look at an old concept. Standard Reduction Potential Values Metallic.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Standard Reduction Potential Values Metallic in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. The following table provides eo for selected reduction. The following table provides eo and eo ´ values for. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard. Standard Reduction Potential Values Metallic.
From byjus.com
the s†an dard reduction potential value of three metallic cations x,y Standard Reduction Potential Values Metallic The value of the reduction under standard conditions (1 bar or 1 atm for gases; 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new. Standard Reduction Potential Values Metallic.
From chemistrytalk.org
Standard Reduction Potentials made easy ChemTalk Standard Reduction Potential Values Metallic standard reduction potential (e°): Standard reduction potentials by element. The following table provides eo and eo ´ values for. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value. The value of the. Standard Reduction Potential Values Metallic.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Values Metallic The following table provides eo for selected reduction. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Standard reduction potentials. Standard Reduction Potential Values Metallic.
From www.vrogue.co
Standard Reduction Potentials Redox Reactions And Ele vrogue.co Standard Reduction Potential Values Metallic standard reduction potential (e°): The following table provides eo for selected reduction. Standard reduction potentials by element. The value of the reduction under standard conditions (1 bar or 1 atm for gases; 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. the standard reduction potential is. Standard Reduction Potential Values Metallic.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Values Metallic standard reduction potential (e°): The following table provides eo for selected reduction. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. The value of the reduction. Standard Reduction Potential Values Metallic.
From www.researchgate.net
Gibbs free energy changes and standard reduction potentials at 25 • C Standard Reduction Potential Values Metallic The value of the reduction under standard conditions (1 bar or 1 atm for gases; the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. 1 m for solutes) usually at 298.15 k;. in this video, we're gonna take a look at an old concept of. Standard Reduction Potential Values Metallic.
From exodxlskk.blob.core.windows.net
Standard Reduction Potential Of Zn2+/Zn at James Tarver blog Standard Reduction Potential Values Metallic standard reduction potential (e°): in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Standard reduction potentials by element. The following. Standard Reduction Potential Values Metallic.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Reduction Potential Values Metallic 1 m for solutes) usually at 298.15 k;. standard reduction potential (e°): The value of the reduction under standard conditions (1 bar or 1 atm for gases; The following table provides eo and eo ´ values for. Standard reduction potentials by element. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard. Standard Reduction Potential Values Metallic.
From www.pinterest.co.kr
Standard Reduction Potential (E) when given two half reactions and Standard Reduction Potential Values Metallic the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The following table provides eo for selected reduction. The following table provides eo and eo ´ values for. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode. Standard Reduction Potential Values Metallic.
From www.researchgate.net
(a) The standard redox potentials of various candidate redox pairs Standard Reduction Potential Values Metallic in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. The following table provides eo for selected reduction. The following table provides eo and eo ´. Standard Reduction Potential Values Metallic.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Values Metallic Standard reduction potentials by value. 1 m for solutes) usually at 298.15 k;. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. The value of. Standard Reduction Potential Values Metallic.
From exoeelsdz.blob.core.windows.net
Standard Electrode Potentials Chemguide at Richard Reddish blog Standard Reduction Potential Values Metallic Standard reduction potentials by element. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. The following table provides eo and eo ´ values. Standard Reduction Potential Values Metallic.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Values Metallic standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. standard reduction potential (e°): 372 rows the data below tabulates standard electrode potentials (e°),. Standard Reduction Potential Values Metallic.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Values Metallic The value of the reduction under standard conditions (1 bar or 1 atm for gases; in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. 1 m for solutes) usually at 298.15 k;. The following table provides eo for selected reduction. Standard reduction potentials by. Standard Reduction Potential Values Metallic.
From www.researchgate.net
Values of the standard potentials of cerium redox systems in anodic Standard Reduction Potential Values Metallic 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine. Standard Reduction Potential Values Metallic.
From labbyag.es
Reduction Potential Chart Labb by AG Standard Reduction Potential Values Metallic Standard reduction potentials by value. the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Standard reduction potentials by element. standard reduction potential (e°): The value of the reduction under standard conditions (1 bar or 1 atm for gases; 1 m for solutes) usually at 298.15. Standard Reduction Potential Values Metallic.
From exoeelsdz.blob.core.windows.net
Standard Electrode Potentials Chemguide at Richard Reddish blog Standard Reduction Potential Values Metallic standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. Standard reduction potentials by value. The value of the reduction under standard conditions (1 bar or 1 atm for gases; Standard reduction potentials by element. 1 m for solutes) usually at 298.15 k;. standard reduction potential (e°): 372 rows. Standard Reduction Potential Values Metallic.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Values Metallic standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. 1 m for solutes) usually at 298.15 k;. Standard reduction potentials by value. The following table. Standard Reduction Potential Values Metallic.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Values Metallic standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. standard reduction potential (e°): The following table provides eo for selected reduction. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard reduction potentials by value. The value. Standard Reduction Potential Values Metallic.
From askfilo.com
The standard reduction potential value of three metallic cations X,Y and Standard Reduction Potential Values Metallic standard reduction potential (e°): 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. the standard reduction potential is the tendency for. Standard Reduction Potential Values Metallic.
From www.pinterest.com
pe=log[e] measuved in volts.if pe is negative reduced, if pe is Standard Reduction Potential Values Metallic in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. The following table provides eo for selected reduction. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. Standard reduction potentials by element. The following table. Standard Reduction Potential Values Metallic.
From www.youtube.com
Lecture 4.6 reduction potentials into Table J YouTube Standard Reduction Potential Values Metallic the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Standard reduction potentials by element. Standard reduction potentials by value. standard reduction potentials (srps) are values that reflect the tendency of a chemical species to gain electrons and. The following table provides eo and eo ´. Standard Reduction Potential Values Metallic.
From askfilo.com
The standard reduction potential value of three metallic cations X,Y and Standard Reduction Potential Values Metallic in this video, we're gonna take a look at an old concept of oxidation and reduction and now combine it with a new variable. 1 m for solutes) usually at 298.15 k;. The value of the reduction under standard conditions (1 bar or 1 atm for gases; standard reduction potentials (srps) are values that reflect the tendency of. Standard Reduction Potential Values Metallic.
From www.flinnsci.ca
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Values Metallic standard reduction potential (e°): The following table provides eo for selected reduction. The following table provides eo and eo ´ values for. Standard reduction potentials by element. 1 m for solutes) usually at 298.15 k;. The value of the reduction under standard conditions (1 bar or 1 atm for gases; 372 rows the data below tabulates standard electrode. Standard Reduction Potential Values Metallic.