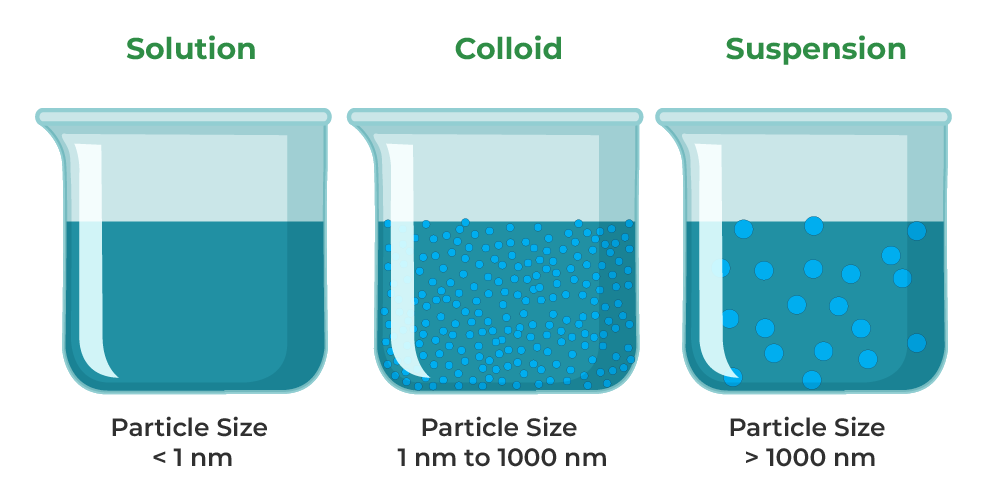
Suspension And Colloid Difference . a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Otherwise, both are heterogeneous mixtures. So, the particles in a suspension typically settle. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. the key difference between a suspension and a colloid is the particle size. the key difference between a suspension and a colloid is that suspensions are.
from www.geeksforgeeks.org
Otherwise, both are heterogeneous mixtures. the key difference between a suspension and a colloid is that suspensions are. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. the key difference between a suspension and a colloid is the particle size. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. So, the particles in a suspension typically settle. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid.
Suspension(Chemistry) Definition, Properties, Examples, and FAQs
Suspension And Colloid Difference So, the particles in a suspension typically settle. the key difference between a suspension and a colloid is that suspensions are. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. Otherwise, both are heterogeneous mixtures. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. So, the particles in a suspension typically settle. the key difference between a suspension and a colloid is the particle size. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid.
From chemwiki.ucdavis.edu
Figure 1 Examples of a stable and of an unstable colloidal dispersion Suspension And Colloid Difference a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. So, the particles in a suspension typically settle. a colloid is a heterogeneous mixture in. Suspension And Colloid Difference.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Suspension And Colloid Difference suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. the key difference between a suspension and a colloid is that suspensions are. So, the particles in a suspension typically settle. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate. Suspension And Colloid Difference.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension And Colloid Difference So, the particles in a suspension typically settle. the key difference between a suspension and a colloid is that suspensions are. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. difference between a colloid and a suspension the particles in a suspension are larger than in a. Suspension And Colloid Difference.
From www.thoughtco.com
Tyndall Effect Definition and Examples Suspension And Colloid Difference suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. difference between a colloid and a suspension the particles in a suspension are larger than. Suspension And Colloid Difference.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Suspension And Colloid Difference So, the particles in a suspension typically settle. the key difference between a suspension and a colloid is that suspensions are. the key difference between a suspension and a colloid is the particle size. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. a colloid is. Suspension And Colloid Difference.
From www.differencebetween.net
Difference Between Colloid and Suspension Difference Between Suspension And Colloid Difference a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. difference between a colloid and a suspension the particles in a suspension are larger than. Suspension And Colloid Difference.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension And Colloid Difference the key difference between a suspension and a colloid is that suspensions are. the key difference between a suspension and a colloid is the particle size. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. suspensions and colloids are two common types of mixtures whose properties. Suspension And Colloid Difference.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Suspension And Colloid Difference difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. the key difference between a suspension and a colloid is that suspensions are. the key difference between a suspension and a colloid is the particle size. suspensions and colloids are two common types of mixtures whose properties are. Suspension And Colloid Difference.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Suspension And Colloid Difference the key difference between a suspension and a colloid is that suspensions are. So, the particles in a suspension typically settle. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples. Suspension And Colloid Difference.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Suspension And Colloid Difference here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. Suspension And Colloid Difference.
From edurev.in
Difference between true solution, suspension and colloidal sol Suspension And Colloid Difference a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. So, the particles in a suspension typically settle. a colloid is a heterogeneous mixture in. Suspension And Colloid Difference.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension And Colloid Difference the key difference between a suspension and a colloid is that suspensions are. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. suspensions and colloids are two common. Suspension And Colloid Difference.
From www.differencebetween.com
Difference Between Colloid and Emulsion Compare the Difference Suspension And Colloid Difference So, the particles in a suspension typically settle. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Otherwise, both are heterogeneous mixtures. the key difference between a suspension and a colloid is the particle size. difference between a colloid and a suspension the particles in a suspension. Suspension And Colloid Difference.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Suspension And Colloid Difference the key difference between a suspension and a colloid is the particle size. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. the key difference between a. Suspension And Colloid Difference.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Suspension And Colloid Difference a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. difference between a colloid and a suspension the particles in a suspension are larger than. Suspension And Colloid Difference.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Suspension And Colloid Difference difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. the key difference between a suspension and a colloid is the particle size. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. the key difference between a suspension. Suspension And Colloid Difference.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Suspension And Colloid Difference the key difference between a suspension and a colloid is the particle size. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. So, the particles in a suspension. Suspension And Colloid Difference.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Suspension And Colloid Difference the key difference between a suspension and a colloid is the particle size. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. the key difference between a. Suspension And Colloid Difference.
From brainly.in
How are colloids and suspensions different from solutions ? Brainly.in Suspension And Colloid Difference Otherwise, both are heterogeneous mixtures. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. the key difference between a suspension and a colloid is the particle size. . Suspension And Colloid Difference.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension And Colloid Difference a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. the key difference between a suspension and a colloid is that suspensions are. a colloid is a heterogeneous mixture. Suspension And Colloid Difference.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Suspension And Colloid Difference the key difference between a suspension and a colloid is that suspensions are. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Otherwise, both are heterogeneous mixtures. . Suspension And Colloid Difference.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Suspension And Colloid Difference suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with. Suspension And Colloid Difference.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension And Colloid Difference the key difference between a suspension and a colloid is that suspensions are. the key difference between a suspension and a colloid is the particle size. Otherwise, both are heterogeneous mixtures. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. a colloid is a mixture in which. Suspension And Colloid Difference.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Suspension And Colloid Difference the key difference between a suspension and a colloid is that suspensions are. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. difference between a colloid and. Suspension And Colloid Difference.
From www.studypage.in
True Solution, Colloidal Solution and Suspension Study Page Suspension And Colloid Difference a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. the key difference between a suspension and a colloid is that suspensions are. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Otherwise, both are heterogeneous mixtures. . Suspension And Colloid Difference.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Suspension And Colloid Difference suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between. Suspension And Colloid Difference.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Suspension And Colloid Difference a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Otherwise, both are heterogeneous mixtures. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. So, the particles in a suspension typically settle. the key. Suspension And Colloid Difference.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension And Colloid Difference suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. the key difference between a suspension and a colloid is that suspensions are. a colloid. Suspension And Colloid Difference.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension And Colloid Difference difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. So, the particles in a suspension typically settle. Otherwise, both are heterogeneous mixtures. the key difference. Suspension And Colloid Difference.
From materialcampuseichel.z19.web.core.windows.net
Solutions Colloids And Suspensions Ppt Suspension And Colloid Difference difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. the key difference between a suspension and a colloid is that suspensions are. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Otherwise, both are heterogeneous mixtures. suspensions. Suspension And Colloid Difference.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Suspension And Colloid Difference the key difference between a suspension and a colloid is the particle size. the key difference between a suspension and a colloid is that suspensions are. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. a colloid is a heterogeneous mixture in. Suspension And Colloid Difference.
From www.slideshare.net
Solutions, suspensions, and colloids Suspension And Colloid Difference suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with. Suspension And Colloid Difference.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspension And Colloid Difference Otherwise, both are heterogeneous mixtures. the key difference between a suspension and a colloid is that suspensions are. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. difference between a colloid and a suspension the particles in a suspension are larger than in a colloid. suspensions. Suspension And Colloid Difference.
From byjus.com
What is difference between true solution and suspension and colloid? Suspension And Colloid Difference a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. the key difference between a suspension and a colloid is that suspensions are. So, the particles in a suspension typically settle. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between. Suspension And Colloid Difference.
From www.slideshare.net
Colloids Suspension And Colloid Difference Otherwise, both are heterogeneous mixtures. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. the key difference between a suspension and a colloid is that suspensions are. a colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. . Suspension And Colloid Difference.