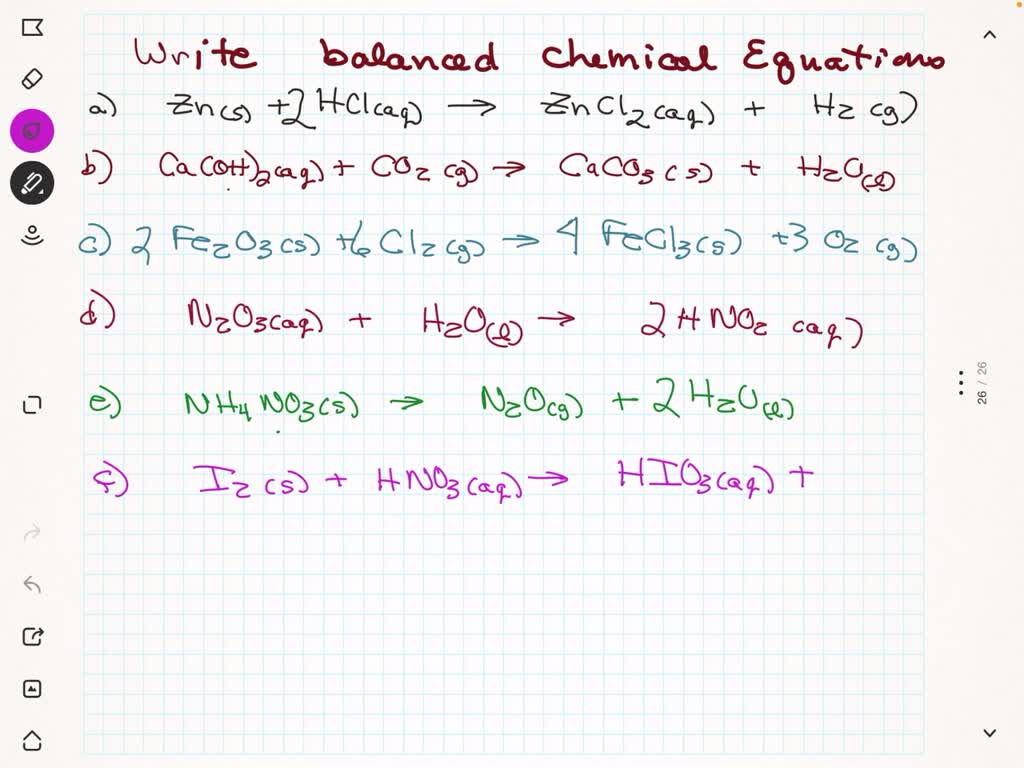
Zinc Carbonate Sulfuric Acid Ionic Equation . Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. enter an equation of an ionic chemical equation and press the balance button. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. first, we set all coefficients to 1: the ionic equation for the reaction. most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation. For each element, we check if the number of atoms. The balanced equation will be calculated along. compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &.
from edu.svet.gob.gt
the ionic equation for the reaction. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. enter an equation of an ionic chemical equation and press the balance button. compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. For each element, we check if the number of atoms. first, we set all coefficients to 1: Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation.
Zinc Carbonate Plus Sulfuric Acid edu.svet.gob.gt
Zinc Carbonate Sulfuric Acid Ionic Equation compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. enter an equation of an ionic chemical equation and press the balance button. the ionic equation for the reaction. compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. first, we set all coefficients to 1: note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. For each element, we check if the number of atoms. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. The balanced equation will be calculated along. most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation.
From diya-jolpblogcooper.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid Zinc Carbonate Sulfuric Acid Ionic Equation most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. note that the reaction between a metal hydroxide and an acid can be represented by. Zinc Carbonate Sulfuric Acid Ionic Equation.
From slideplayer.com
Barium Hydroxide Sulfuric Acid Reaction ppt download Zinc Carbonate Sulfuric Acid Ionic Equation compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. For each element, we check if the number of atoms. Kbr (s) → k + (aq) + br − (aq) not only do. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.tessshebaylo.com
Sodium Carbonate Plus Hydrochloric Acid Complete Ionic Equation Zinc Carbonate Sulfuric Acid Ionic Equation most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation. first, we set all coefficients to 1: 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. All carbonates react in the same sort of way and. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.numerade.com
SOLVED 16. Mixing each pair of aqueous solutions results in a chemical Zinc Carbonate Sulfuric Acid Ionic Equation For each element, we check if the number of atoms. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. The balanced equation will be calculated along. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.nagwa.com
Question Video Writing a Net Ionic Equation for the Reaction of Solid Zinc Carbonate Sulfuric Acid Ionic Equation Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. The balanced equation will be calculated along. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. . Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.chemicals.co.uk
Practical GCSE Chemistry Making Salts The Science Blog Zinc Carbonate Sulfuric Acid Ionic Equation 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.numerade.com
SOLVED(e) Calcium carbonate and perchloric acid Molecular equation Zinc Carbonate Sulfuric Acid Ionic Equation compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation. For each element, we check if the number of atoms. the ionic equation for the reaction. All carbonates react in. Zinc Carbonate Sulfuric Acid Ionic Equation.
From edu.svet.gob.gt
Zinc Carbonate Plus Sulfuric Acid edu.svet.gob.gt Zinc Carbonate Sulfuric Acid Ionic Equation All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. the ionic equation for the reaction. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. For. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.toppr.com
Balanced equation for the reaction between zinc and dilute sulphuric Zinc Carbonate Sulfuric Acid Ionic Equation All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. For each element, we check if the number of atoms. most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation. first, we set all coefficients. Zinc Carbonate Sulfuric Acid Ionic Equation.
From giolompon.blob.core.windows.net
Iron Iii Chloride Sodium Hydroxide Net Ionic Equation at Fran Swain blog Zinc Carbonate Sulfuric Acid Ionic Equation Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. All carbonates react in the same sort of. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.slideserve.com
PPT Net Ionic equations PowerPoint Presentation, free download ID Zinc Carbonate Sulfuric Acid Ionic Equation Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. For each element, we check if the number of atoms. enter an equation of an ionic chemical equation and press the balance button. most of the acid. Zinc Carbonate Sulfuric Acid Ionic Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Zinc Carbonate Sulfuric Acid Ionic Equation Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. note that the reaction between a metal. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.dreamstime.com
Sulfuric Acid Strong Mineral Acid Molecule. Skeletal Formula Stock Zinc Carbonate Sulfuric Acid Ionic Equation first, we set all coefficients to 1: note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. enter an equation of an ionic chemical equation and press the balance button. Kbr (s) → k + (aq) + br − (aq) not only do. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.toppr.com
Balanced equation for the reaction between zinc and dilute sulphuric Zinc Carbonate Sulfuric Acid Ionic Equation For each element, we check if the number of atoms. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. 1 znso 4 + 1 h 2 co 3 = 1 znco 3. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.numerade.com
SOLVED A. Write a net ionic equation for the reaction that occurs when Zinc Carbonate Sulfuric Acid Ionic Equation compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. the ionic equation for the reaction. The balanced equation will be calculated along. most of the acid. Zinc Carbonate Sulfuric Acid Ionic Equation.
From edu.svet.gob.gt
Zinc Carbonate Plus Sulfuric Acid edu.svet.gob.gt Zinc Carbonate Sulfuric Acid Ionic Equation the ionic equation for the reaction. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. enter an equation of an ionic chemical equation and press the balance button. For each element, we check if the number of atoms. Kbr (s) → k + (aq) + br − (aq). Zinc Carbonate Sulfuric Acid Ionic Equation.
From tia-jolpblogramsey.blogspot.com
Zinc Carbonate Sulfuric Acid Zinc Carbonate Sulfuric Acid Ionic Equation enter an equation of an ionic chemical equation and press the balance button. first, we set all coefficients to 1: 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.coursehero.com
[Solved] What ionic reaction formula occurs between sodium carbonate Zinc Carbonate Sulfuric Acid Ionic Equation compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. enter an equation of an ionic chemical equation and press the balance button. most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation. All carbonates react in the same sort. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.chegg.com
Solved Choose balanced molecular, ionic, and net ionic Zinc Carbonate Sulfuric Acid Ionic Equation most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation. enter an equation of an ionic chemical equation and press the balance button. the ionic equation for the reaction. For each element, we check if the number of atoms. 1 znso 4 + 1 h. Zinc Carbonate Sulfuric Acid Ionic Equation.
From studygripewater.z21.web.core.windows.net
Sulfuric Acid Formation Zinc Carbonate Sulfuric Acid Ionic Equation compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. the ionic equation for the reaction. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. Kbr (s) → k + (aq) + br − (aq) not only. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.tessshebaylo.com
Sodium Carbonate And Hydrochloric Acid Net Ionic Equation Tessshebaylo Zinc Carbonate Sulfuric Acid Ionic Equation first, we set all coefficients to 1: the ionic equation for the reaction. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. 1 znso 4 + 1 h 2 co. Zinc Carbonate Sulfuric Acid Ionic Equation.
From breathcareer14.pythonanywhere.com
ppt acids bases powerpoint presentation free download id 2732225 Zinc Carbonate Sulfuric Acid Ionic Equation enter an equation of an ionic chemical equation and press the balance button. first, we set all coefficients to 1: For each element, we check if the number of atoms. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. compute answers using wolfram's breakthrough technology. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Na2S + Zn(NO3)2 = NaNO3 + ZnS Zinc Carbonate Sulfuric Acid Ionic Equation All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. enter an equation of an ionic chemical equation and press the balance button. most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation. the. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.chegg.com
Solved Write the net ionic equation for the reaction between Zinc Carbonate Sulfuric Acid Ionic Equation note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. The balanced equation will be calculated along. the ionic equation for the reaction. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. compute. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Carbonate Sulfuric Acid Ionic Equation note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. Kbr (s) → k + (aq) + br − (aq) not only do the. Zinc Carbonate Sulfuric Acid Ionic Equation.
From hxeeqzkvm.blob.core.windows.net
Zinc Carbonate + Nitric Acid at Danielle McCormick blog Zinc Carbonate Sulfuric Acid Ionic Equation 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium. Zinc Carbonate Sulfuric Acid Ionic Equation.
From tia-jolpblogramsey.blogspot.com
Zinc Carbonate Sulfuric Acid Zinc Carbonate Sulfuric Acid Ionic Equation note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. The balanced equation will be calculated along. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. All carbonates react in the same sort of way. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.slideshare.net
Acids And Bases Zinc Carbonate Sulfuric Acid Ionic Equation enter an equation of an ionic chemical equation and press the balance button. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. For each element, we check if. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.equationsworksheets.net
Acid Base Net Ionic Equation Worksheet Equations Worksheets Zinc Carbonate Sulfuric Acid Ionic Equation All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. most of the acid molecules are not ionized, so you must write out. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.tessshebaylo.com
Chemical Equation For Sodium Hydrogen Carbonate And Hydrochloric Acid Zinc Carbonate Sulfuric Acid Ionic Equation most of the acid molecules are not ionized, so you must write out the complete formula of the acid in your equation. For each element, we check if the number of atoms. the ionic equation for the reaction. The balanced equation will be calculated along. All carbonates react in the same sort of way and that is because. Zinc Carbonate Sulfuric Acid Ionic Equation.
From childhealthpolicy.vumc.org
💄 Calcium carbonate reacts with hydrochloric acid balanced equation Zinc Carbonate Sulfuric Acid Ionic Equation compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. For each element, we check if the number of atoms. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. All carbonates react in the same sort of way. Zinc Carbonate Sulfuric Acid Ionic Equation.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Zinc Carbonate Sulfuric Acid Ionic Equation compute answers using wolfram's breakthrough technology & knowledgebase, relied on by millions of students &. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in. enter an equation of an ionic chemical equation and press the balance button. For each element, we check if the number of. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.nagwa.com
Question Video Identifying the Name of Ions that Are Not Included in Zinc Carbonate Sulfuric Acid Ionic Equation The balanced equation will be calculated along. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and the. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. For each element, we check if the number of. Zinc Carbonate Sulfuric Acid Ionic Equation.
From aliananewsgaines.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid Zinc Carbonate Sulfuric Acid Ionic Equation the ionic equation for the reaction. For each element, we check if the number of atoms. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. note that the reaction between a metal hydroxide and an acid. Zinc Carbonate Sulfuric Acid Ionic Equation.
From www.tessshebaylo.com
Sodium Carbonate And Hydrobromic Acid Net Ionic Equation Tessshebaylo Zinc Carbonate Sulfuric Acid Ionic Equation The balanced equation will be calculated along. For each element, we check if the number of atoms. 1 znso 4 + 1 h 2 co 3 = 1 znco 3 + 1 h 2 so 4. note that the reaction between a metal hydroxide and an acid can be represented by an ionic equation between the hydrogen ions and. Zinc Carbonate Sulfuric Acid Ionic Equation.