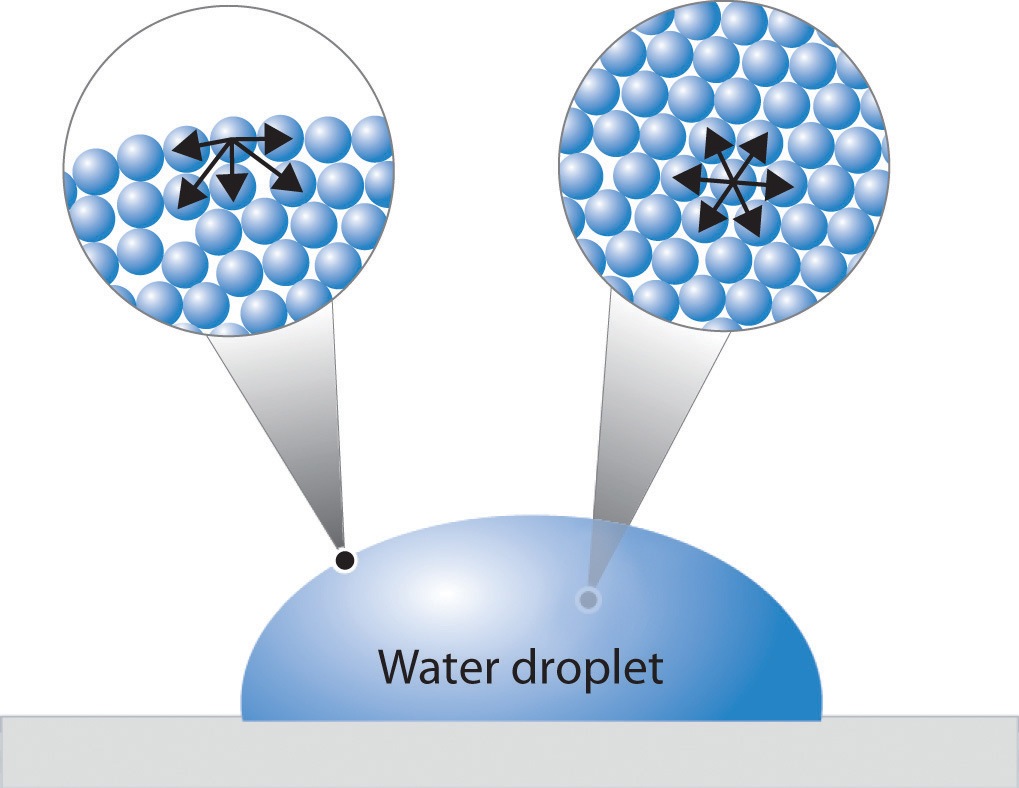
How Would Surface Tension Of Water Be Increased . Water striders) to float on a. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. The stronger the intermolecular interactions, the greater the surface tension. The surface tension will decrease if the impurities are less soluble (for example,. As temperature decreases, surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The presence of soluble particles in water may increase or decrease surface tension. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Let’s consider the effects of these four conditions on surface tension: Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface.
from chem.libretexts.org
Water striders) to float on a. Surface tension is the energy required to increase the surface area of a liquid by a given amount. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. As temperature decreases, surface tension. Let’s consider the effects of these four conditions on surface tension: Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The surface tension will decrease if the impurities are less soluble (for example,. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f).
Surface Tension Chemistry LibreTexts
How Would Surface Tension Of Water Be Increased Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. The surface tension will decrease if the impurities are less soluble (for example,. Let’s consider the effects of these four conditions on surface tension: The presence of soluble particles in water may increase or decrease surface tension. The stronger the intermolecular interactions, the greater the surface tension. As temperature decreases, surface tension. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Water striders) to float on a. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ).
From www.biolinscientific.com
Surface tension of water Why is it so high? How Would Surface Tension Of Water Be Increased Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The surface tension will decrease if the impurities are less soluble (for example,. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Surface tension in water might be good at performing tricks, such as being able to float. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID2046092 How Would Surface Tension Of Water Be Increased The surface tension will decrease if the impurities are less soluble (for example,. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Water striders) to float on a. As temperature decreases, surface tension. The stronger the intermolecular interactions, the greater the surface tension. Surface tension of water can cause things. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free download ID6578004 How Would Surface Tension Of Water Be Increased Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Surface. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 How Would Surface Tension Of Water Be Increased As temperature decreases, surface tension. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The stronger the intermolecular interactions, the greater the surface tension. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Let’s consider the effects of these four conditions on surface. How Would Surface Tension Of Water Be Increased.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Would Surface Tension Of Water Be Increased The presence of soluble particles in water may increase or decrease surface tension. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The surface tension will decrease if the impurities are less soluble (for example,. Surface tension in water might be good at performing tricks, such as being able to float a paper. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 How Would Surface Tension Of Water Be Increased Water striders) to float on a. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy required to increase the surface area of a liquid by a given amount. As temperature decreases, surface tension. The stronger the intermolecular interactions, the greater the surface tension. Surface tension in water might. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Would Surface Tension Of Water Be Increased In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Water striders) to float on a. The surface tension will decrease if the impurities are less soluble (for example,. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. The stronger the intermolecular interactions, the greater the surface. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download ID2019622 How Would Surface Tension Of Water Be Increased Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water striders) to float on a. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The stronger the intermolecular interactions, the greater the surface tension. As temperature decreases, surface tension. In comparison, organic liquids, such. How Would Surface Tension Of Water Be Increased.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Transcript How Would Surface Tension Of Water Be Increased Water striders) to float on a. The presence of soluble particles in water may increase or decrease surface tension. The stronger the intermolecular interactions, the greater the surface tension. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The surface tension will decrease if the impurities are less soluble (for example,. Surface tension. How Would Surface Tension Of Water Be Increased.
From byjus.com
Explain the surface tension phenomenon with examples. How Would Surface Tension Of Water Be Increased In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The surface tension will decrease if the impurities are less soluble (for example,. Let’s consider the effects of these four conditions on surface tension: Water has a surface tension. How Would Surface Tension Of Water Be Increased.
From ar.inspiredpencil.com
Surface Tension Of Water Diagram How Would Surface Tension Of Water Be Increased In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. As temperature decreases, surface tension. The surface tension will decrease if the impurities are less soluble (for example,. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Water striders) to float on. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Presentation ID3374064 How Would Surface Tension Of Water Be Increased Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. Surface tension in water might be good at performing tricks, such as being able to float a paper. How Would Surface Tension Of Water Be Increased.
From byjus.com
17. In a capillary tube containing water how does the surface tension force acts in which How Would Surface Tension Of Water Be Increased The surface tension will decrease if the impurities are less soluble (for example,. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Surfactants are molecules, such. How Would Surface Tension Of Water Be Increased.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse How Would Surface Tension Of Water Be Increased Let’s consider the effects of these four conditions on surface tension: Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. The presence of soluble particles in water may increase. How Would Surface Tension Of Water Be Increased.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co How Would Surface Tension Of Water Be Increased Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The presence of soluble particles in water may increase or decrease surface tension. As temperature decreases, surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Surface tension. How Would Surface Tension Of Water Be Increased.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces between water molecules How Would Surface Tension Of Water Be Increased Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. The presence of soluble particles in water may increase or decrease surface tension. Water striders) to float on a. As temperature decreases, surface tension. The stronger the intermolecular interactions, the greater the surface tension. Surface tension. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID2755903 How Would Surface Tension Of Water Be Increased As temperature decreases, surface tension. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. The stronger the intermolecular interactions, the greater the surface tension. Let’s consider the effects of these four conditions on surface tension: Surface tension of. How Would Surface Tension Of Water Be Increased.
From www.youtube.com
DETERMINING THE SURFACE TENSION OF WATER EASY TO UNDERSTAND YouTube How Would Surface Tension Of Water Be Increased In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. The stronger the intermolecular interactions, the greater the surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Water striders) to float on a. Let’s consider the effects of these four. How Would Surface Tension Of Water Be Increased.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it impart YouTube How Would Surface Tension Of Water Be Increased Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The presence of soluble particles in water may increase or decrease surface tension. Water has a surface tension of 0.07275 joule per square metre. How Would Surface Tension Of Water Be Increased.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? How Would Surface Tension Of Water Be Increased As temperature decreases, surface tension. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Let’s consider the effects of these four conditions on surface tension: Surface tension in water might be good at performing tricks,. How Would Surface Tension Of Water Be Increased.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension How Would Surface Tension Of Water Be Increased Water striders) to float on a. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. As temperature decreases, surface tension. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Surfactants are molecules, such as soaps and detergents, that reduce the surface. How Would Surface Tension Of Water Be Increased.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Would Surface Tension Of Water Be Increased Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The surface tension will decrease if the impurities are less soluble (for example,. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,.. How Would Surface Tension Of Water Be Increased.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Would Surface Tension Of Water Be Increased As temperature decreases, surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy required to increase the surface area of a liquid by. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download ID6877497 How Would Surface Tension Of Water Be Increased Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Would Surface Tension Of Water Be Increased Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. Let’s. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Presentation ID3525649 How Would Surface Tension Of Water Be Increased Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. Surface tension in water might be good at performing tricks, such as being. How Would Surface Tension Of Water Be Increased.
From www.youtube.com
Surface Tension of Water Explained YouTube How Would Surface Tension Of Water Be Increased Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. As temperature decreases, surface tension. Surface tension is what allows objects with a higher density than water such as razor blades and insects. How Would Surface Tension Of Water Be Increased.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii How Would Surface Tension Of Water Be Increased Water striders) to float on a. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The presence of soluble particles in water may increase or decrease surface tension. Let’s consider the effects of these four conditions on surface tension: The surface tension will decrease if the impurities are less soluble (for. How Would Surface Tension Of Water Be Increased.
From www.youtube.com
To study surface tension of water by capillary rise method. YouTube How Would Surface Tension Of Water Be Increased Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The surface tension will decrease if the impurities are less soluble (for example,. Surface tension in water might be good at performing tricks, such as being. How Would Surface Tension Of Water Be Increased.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Would Surface Tension Of Water Be Increased Let’s consider the effects of these four conditions on surface tension: The stronger the intermolecular interactions, the greater the surface tension. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Surface tension of water can cause things. How Would Surface Tension Of Water Be Increased.
From www.linkedin.com
HOW NANOBUBBLES IMPROVE WATER INFILTRATION AND BY REDUCING THE SURFACE TENSION OF WATER How Would Surface Tension Of Water Be Increased Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Surface tension is what allows objects with a higher density than water such as razor blades and insects. How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Chapter 15 “Water and Aqueous Systems” PowerPoint Presentation ID2915263 How Would Surface Tension Of Water Be Increased Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. The stronger the intermolecular interactions, the greater the surface tension. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The presence of soluble particles in water may. How Would Surface Tension Of Water Be Increased.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical Science, Science Lesson Plans How Would Surface Tension Of Water Be Increased The surface tension will decrease if the impurities are less soluble (for example,. The presence of soluble particles in water may increase or decrease surface tension. Let’s consider the effects of these four conditions on surface tension: Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\). How Would Surface Tension Of Water Be Increased.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation ID2778612 How Would Surface Tension Of Water Be Increased Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. Let’s consider the effects of these four conditions on surface tension: In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. As temperature decreases, surface tension. The surface tension will decrease if the impurities are less soluble (for example,.. How Would Surface Tension Of Water Be Increased.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Would Surface Tension Of Water Be Increased The presence of soluble particles in water may increase or decrease surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Let’s consider the effects of these four conditions on surface tension: Surface tension of water can cause things to float which are denser than water, allowing organisms to literally. How Would Surface Tension Of Water Be Increased.