Liquid Surface Tension Due To Which Molecular Force . This general effect is called. surface tension is the energy required to increase the surface area of a liquid by a given amount. This general effect is called. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The molecules on the surface of a liquid are. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.
from www.expii.com
Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called. The molecules on the surface of a liquid are. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. This general effect is called. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or.
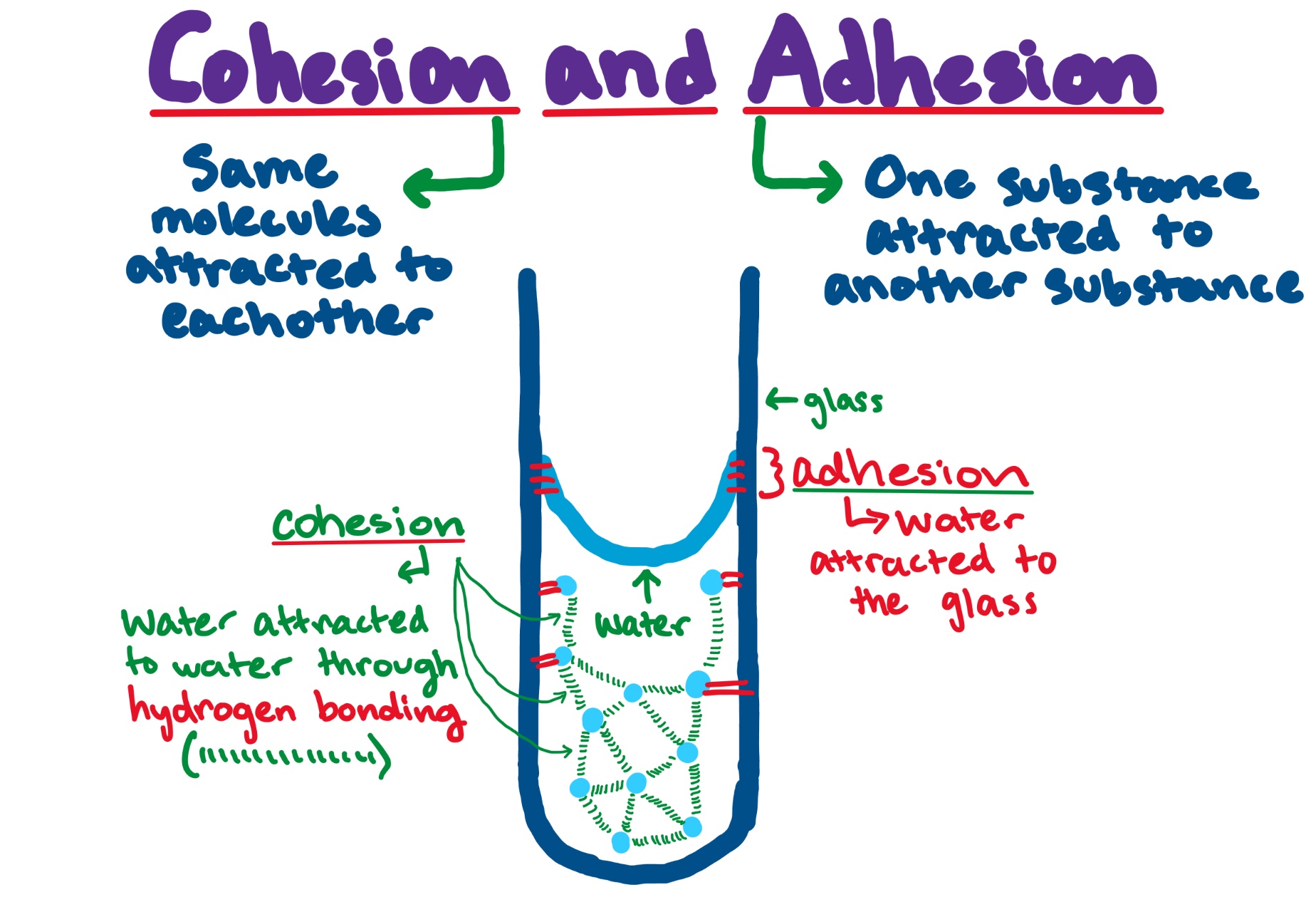
Cohesion and Adhesion — Definition & Overview Expii
Liquid Surface Tension Due To Which Molecular Force Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules.
From www.expii.com
Cohesion and Adhesion — Definition & Overview Expii Liquid Surface Tension Due To Which Molecular Force surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The molecules on the surface of a liquid are. This general effect is called. surface tension is the energy. Liquid Surface Tension Due To Which Molecular Force.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Liquid Surface Tension Due To Which Molecular Force Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy, or work, required to increase the surface area of. Liquid Surface Tension Due To Which Molecular Force.
From www.researchgate.net
Surface tension force at the gas/liquid interface of a bubble Liquid Surface Tension Due To Which Molecular Force surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This general effect is called. The molecules on the surface of a liquid are. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension depends mainly upon the. Liquid Surface Tension Due To Which Molecular Force.
From chem.libretexts.org
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts Liquid Surface Tension Due To Which Molecular Force This general effect is called. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension depends mainly upon the forces of attraction between the particles within the given. Liquid Surface Tension Due To Which Molecular Force.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Liquid Surface Tension Due To Which Molecular Force Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension depends mainly upon the forces of attraction between the particles within. Liquid Surface Tension Due To Which Molecular Force.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Liquid Surface Tension Due To Which Molecular Force This general effect is called. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. This general effect is called. Cohesive forces between molecules cause. Liquid Surface Tension Due To Which Molecular Force.
From byjus.com
Explain surface tension on the basis of molecular theory. Liquid Surface Tension Due To Which Molecular Force surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest. Liquid Surface Tension Due To Which Molecular Force.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation Liquid Surface Tension Due To Which Molecular Force Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called. surface tension is the energy required to increase the surface area of a liquid by a given amount.. Liquid Surface Tension Due To Which Molecular Force.
From brainly.in
1. Define surface tension. Write formula, unit and dimension of surface Liquid Surface Tension Due To Which Molecular Force surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface. Liquid Surface Tension Due To Which Molecular Force.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts Liquid Surface Tension Due To Which Molecular Force surface tension is the energy required to increase the surface area of a liquid by a given amount. This general effect is called. This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to. Liquid Surface Tension Due To Which Molecular Force.
From hxegmgtnx.blob.core.windows.net
Does High Surface Tension Mean Strong Intermolecular Forces at Laura Liquid Surface Tension Due To Which Molecular Force The molecules on the surface of a liquid are. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy required to increase. Liquid Surface Tension Due To Which Molecular Force.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Liquid Surface Tension Due To Which Molecular Force surface tension is the energy required to increase the surface area of a liquid by a given amount. This general effect is called. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. Cohesive forces between molecules cause the surface of a liquid to contract. Liquid Surface Tension Due To Which Molecular Force.
From courses.lumenlearning.com
Properties of Liquids Chemistry Liquid Surface Tension Due To Which Molecular Force surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible. Liquid Surface Tension Due To Which Molecular Force.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement Liquid Surface Tension Due To Which Molecular Force Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy required to increase the surface area of a liquid by a given amount. This general effect is called. This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to. Liquid Surface Tension Due To Which Molecular Force.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid Liquid Surface Tension Due To Which Molecular Force The molecules on the surface of a liquid are. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy required to increase the surface area of a liquid. Liquid Surface Tension Due To Which Molecular Force.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Liquid Surface Tension Due To Which Molecular Force surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. The molecules on the surface of a liquid are. This general effect is called. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension. Liquid Surface Tension Due To Which Molecular Force.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Liquid Surface Tension Due To Which Molecular Force The molecules on the surface of a liquid are. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. This general effect is called. Cohesive forces between molecules cause the. Liquid Surface Tension Due To Which Molecular Force.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Liquid Surface Tension Due To Which Molecular Force surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are. surface tension is. Liquid Surface Tension Due To Which Molecular Force.
From www.youtube.com
Surface Tension, part 1 Lecture 1.3 Chemical Engineering Fluid Liquid Surface Tension Due To Which Molecular Force surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension depends. Liquid Surface Tension Due To Which Molecular Force.
From www.youtube.com
Surface Tension of Water Explained YouTube Liquid Surface Tension Due To Which Molecular Force The molecules on the surface of a liquid are. This general effect is called. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. This general effect is called. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Cohesive. Liquid Surface Tension Due To Which Molecular Force.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Liquid Surface Tension Due To Which Molecular Force surface tension is the energy required to increase the surface area of a liquid by a given amount. The molecules on the surface of a liquid are. This general effect is called. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension is the energy, or work, required to increase. Liquid Surface Tension Due To Which Molecular Force.
From askfilo.com
\ 6 \rightarrow What is surface tension of liquids? Explain the effect o.. Liquid Surface Tension Due To Which Molecular Force surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface. Liquid Surface Tension Due To Which Molecular Force.
From www.biolinscientific.com
Surface tension of water Why is it so high? Liquid Surface Tension Due To Which Molecular Force Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are. surface tension depends mainly upon the forces of attraction between the. Liquid Surface Tension Due To Which Molecular Force.
From giopacdya.blob.core.windows.net
Explain Surface Tension By Completing The Diagram Below at Zachary Liquid Surface Tension Due To Which Molecular Force This general effect is called. The molecules on the surface of a liquid are. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a. Liquid Surface Tension Due To Which Molecular Force.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Liquid Surface Tension Due To Which Molecular Force This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. This general effect. Liquid Surface Tension Due To Which Molecular Force.
From www.youtube.com
12.3 Intermolecular Forces in Action Surface Tension & Viscosity YouTube Liquid Surface Tension Due To Which Molecular Force surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or. This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is a phenomenon that occurs due to the cohesive forces. Liquid Surface Tension Due To Which Molecular Force.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Liquid Surface Tension Due To Which Molecular Force This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also. Liquid Surface Tension Due To Which Molecular Force.
From www.chemistrylibrary.org
Surface Tension Liquid Surface Tension Due To Which Molecular Force This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called. surface tension is a. Liquid Surface Tension Due To Which Molecular Force.
From readchemistry.com
Determination of Surface Tension Read Chemistry Liquid Surface Tension Due To Which Molecular Force Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are. This general effect is called. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension is a phenomenon that occurs due. Liquid Surface Tension Due To Which Molecular Force.
From mackenzie-has-adkins.blogspot.com
Describe What Surface Tension Is Using Theory Liquid Surface Tension Due To Which Molecular Force surface tension is the energy required to increase the surface area of a liquid by a given amount. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are. surface tension depends mainly upon the forces of attraction between the particles within. Liquid Surface Tension Due To Which Molecular Force.
From pressbooks.bccampus.ca
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Liquid Surface Tension Due To Which Molecular Force This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension depends mainly upon the forces of attraction. Liquid Surface Tension Due To Which Molecular Force.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Liquid Surface Tension Due To Which Molecular Force This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension is the energy, or work, required to increase the surface area of a liquid due. Liquid Surface Tension Due To Which Molecular Force.
From www.bigstockphoto.com
Surface Tension Image & Photo (Free Trial) Bigstock Liquid Surface Tension Due To Which Molecular Force surface tension is the energy required to increase the surface area of a liquid by a given amount. This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension depends mainly upon the forces of attraction between the particles within the given liquid and. Liquid Surface Tension Due To Which Molecular Force.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Liquid Surface Tension Due To Which Molecular Force This general effect is called. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy required to increase the surface area of a liquid by a given amount. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.. Liquid Surface Tension Due To Which Molecular Force.
From www.youtube.com
Surface Tension YouTube Liquid Surface Tension Due To Which Molecular Force surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the energy required to increase the surface area of a liquid by a given amount. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general. Liquid Surface Tension Due To Which Molecular Force.