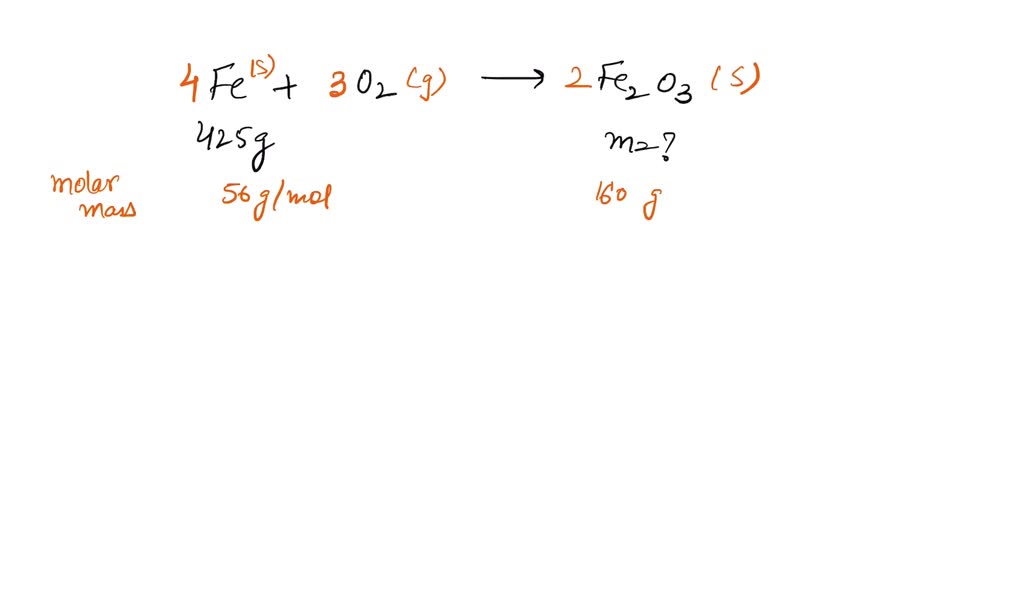Iron Iii Oxide + Magnesium Reaction . the reaction between magnesium and copper (ii) oxide. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). Magnesium oxide and zinc will form. displace iron from iron(iii) oxide. Start by watching this very, very short bit of youtube video. a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. Reduction is the gain of electrons by a substance. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: iron(iii) oxide is reduced because it loses oxygen to form iron. mg + fe2o3 = fe + mgo is a single displacement (substitution) reaction where three moles of magnesium [mg] and one. (d) a reaction will happen. 2mg + o 2 → 2mgo. It is also the loss. This is because magnesium is more. This is one of the classic examples.
from www.numerade.com
magnesium + oxygen → magnesium oxide. (d) a reaction will happen. Cu 2+ + mg cu + mg 2+. 2mg + o 2 → 2mgo. a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. I am going to leave out state symbols in all the. This is one of the classic examples. Start by watching this very, very short bit of youtube video. Magnesium oxide and zinc will form. This is because magnesium is more.
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a
Iron Iii Oxide + Magnesium Reaction when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: This is one of the classic examples. This is because magnesium is more. I am going to leave out state symbols in all the. a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. magnesium + oxygen → magnesium oxide. (d) a reaction will happen. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. displace iron from iron(iii) oxide. iron(iii) oxide is reduced because it loses oxygen to form iron. Reduction is the gain of electrons by a substance. the reaction between magnesium and copper (ii) oxide. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). It is also the loss. Start by watching this very, very short bit of youtube video. Magnesium oxide and zinc will form.
From www.youtube.com
when iron rusts, solid iron reacts with gaseous oxygen to form solid Iron Iii Oxide + Magnesium Reaction (d) a reaction will happen. the reaction between magnesium and copper (ii) oxide. It is also the loss. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: This is because magnesium is more. a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides. Iron Iii Oxide + Magnesium Reaction.
From cerojfxl.blob.core.windows.net
Iron Iii Oxide Examples at Elaine Ortiz blog Iron Iii Oxide + Magnesium Reaction when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: (d) a reaction will happen. displace iron from iron(iii) oxide. Reduction is the gain of electrons by a substance. mg + fe2o3 = fe + mgo is a single displacement (substitution) reaction where three moles of magnesium [mg] and one. Cu. Iron Iii Oxide + Magnesium Reaction.
From www.researchgate.net
Schematic of the principal pathways of iron oxide formation in aqueous Iron Iii Oxide + Magnesium Reaction magnesium + oxygen → magnesium oxide. mg + fe2o3 = fe + mgo is a single displacement (substitution) reaction where three moles of magnesium [mg] and one. It is also the loss. displace iron from iron(iii) oxide. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). the reaction between magnesium and copper (ii). Iron Iii Oxide + Magnesium Reaction.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube Iron Iii Oxide + Magnesium Reaction mg + fe2o3 = fe + mgo is a single displacement (substitution) reaction where three moles of magnesium [mg] and one. 2mg + o 2 → 2mgo. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. I am going to leave out state symbols in all the. This is because. Iron Iii Oxide + Magnesium Reaction.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron Iii Oxide + Magnesium Reaction magnesium + oxygen → magnesium oxide. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: iron(iii) oxide is reduced because it loses oxygen to form iron. It is also the loss. This. Iron Iii Oxide + Magnesium Reaction.
From www.numerade.com
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a Iron Iii Oxide + Magnesium Reaction iron(iii) oxide is reduced because it loses oxygen to form iron. the reaction between magnesium and copper (ii) oxide. displace iron from iron(iii) oxide. magnesium + oxygen → magnesium oxide. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: Start by watching this very, very short bit of. Iron Iii Oxide + Magnesium Reaction.
From mrcolessciencepage.yolasite.com
HRHS Chemistry Iron Iii Oxide + Magnesium Reaction This is because magnesium is more. (d) a reaction will happen. 2mg + o 2 → 2mgo. This is one of the classic examples. iron(iii) oxide is reduced because it loses oxygen to form iron. Reduction is the gain of electrons by a substance. I am going to leave out state symbols in all the. Carbon monoxide acts as. Iron Iii Oxide + Magnesium Reaction.
From cevzuiig.blob.core.windows.net
Iron 3 Oxide State Of Matter at Marcus Ellis blog Iron Iii Oxide + Magnesium Reaction a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. This is one of the classic examples. (d) a reaction will happen. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: displace iron from. Iron Iii Oxide + Magnesium Reaction.
From www.scibond.com
How to represent a chemical reaction? SciBond Iron Iii Oxide + Magnesium Reaction Carbon monoxide acts as a reducing agent because it can reduce iron(iii). Magnesium oxide and zinc will form. This is one of the classic examples. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. Reduction is the gain of electrons by a substance. I am going to leave out state symbols. Iron Iii Oxide + Magnesium Reaction.
From www.coursehero.com
[Solved] Iron (III) oxide is formed when iron combines with oxygen in Iron Iii Oxide + Magnesium Reaction It is also the loss. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: This is because magnesium is more. mg + fe2o3 = fe + mgo is a single displacement (substitution) reaction where three moles of magnesium [mg] and one. Magnesium oxide and zinc will form. in this video. Iron Iii Oxide + Magnesium Reaction.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron Iii Oxide + Magnesium Reaction the reaction between magnesium and copper (ii) oxide. displace iron from iron(iii) oxide. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. mg + fe2o3 = fe + mgo is a single displacement (substitution) reaction where three moles of magnesium [mg] and one. Reduction is the gain of. Iron Iii Oxide + Magnesium Reaction.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron Iii Oxide + Magnesium Reaction Carbon monoxide acts as a reducing agent because it can reduce iron(iii). This is because magnesium is more. Magnesium oxide and zinc will form. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. Reduction is the gain of electrons by a substance. Cu 2+ + mg cu + mg 2+. . Iron Iii Oxide + Magnesium Reaction.
From www.slideshare.net
Reactions & Formulas Iron Iii Oxide + Magnesium Reaction when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: iron(iii) oxide is reduced because it loses oxygen to form iron. 2mg + o 2 → 2mgo. This is one of the classic examples. It is also the loss. in this video we'll balance the equation mg + fe2o3 = fe. Iron Iii Oxide + Magnesium Reaction.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Iron Iii Oxide + Magnesium Reaction This is because magnesium is more. This is one of the classic examples. Magnesium oxide and zinc will form. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: Cu 2+ + mg cu + mg 2+. (d) a reaction will happen. displace iron from iron(iii) oxide. iron(iii) oxide is reduced. Iron Iii Oxide + Magnesium Reaction.
From www.coursehero.com
[Solved] The unbalanced equation for the reaction between iron (III Iron Iii Oxide + Magnesium Reaction Cu 2+ + mg cu + mg 2+. This is one of the classic examples. It is also the loss. (d) a reaction will happen. the reaction between magnesium and copper (ii) oxide. displace iron from iron(iii) oxide. 2mg + o 2 → 2mgo. Reduction is the gain of electrons by a substance. iron(iii) oxide is reduced. Iron Iii Oxide + Magnesium Reaction.
From www.mdpi.com
Free FullText Diversity of Iron Oxides Iron Iii Oxide + Magnesium Reaction a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). Start by watching this very, very short bit of youtube video. displace iron from iron(iii) oxide. magnesium + oxygen. Iron Iii Oxide + Magnesium Reaction.
From ceptbcph.blob.core.windows.net
Iron Iii Oxide And Sulfuric Acid at John Coulter blog Iron Iii Oxide + Magnesium Reaction (d) a reaction will happen. It is also the loss. Start by watching this very, very short bit of youtube video. 2mg + o 2 → 2mgo. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. Cu 2+ + mg cu + mg 2+. Reduction is the gain of electrons by. Iron Iii Oxide + Magnesium Reaction.
From cevzuiig.blob.core.windows.net
Iron 3 Oxide State Of Matter at Marcus Ellis blog Iron Iii Oxide + Magnesium Reaction a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. Magnesium oxide and zinc will form. Reduction is the gain of electrons by a substance. 2mg + o 2 → 2mgo. It is also the loss. the reaction between magnesium and copper. Iron Iii Oxide + Magnesium Reaction.
From www.numerade.com
SOLVED Iron (III) oxide reacts with carbon monoxide to produce iron Iron Iii Oxide + Magnesium Reaction displace iron from iron(iii) oxide. Magnesium oxide and zinc will form. mg + fe2o3 = fe + mgo is a single displacement (substitution) reaction where three moles of magnesium [mg] and one. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). the reaction between magnesium and copper (ii) oxide. This is one of the. Iron Iii Oxide + Magnesium Reaction.
From www.researchgate.net
Scheme 1. The reactions involved in the production of iron oxide Iron Iii Oxide + Magnesium Reaction magnesium + oxygen → magnesium oxide. Magnesium oxide and zinc will form. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). This is because magnesium is more. Cu 2+ + mg cu + mg 2+. This is one of the classic examples. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for. Iron Iii Oxide + Magnesium Reaction.
From dxooogleu.blob.core.windows.net
Iron (Iii) Oxide Type Of Reaction at Katherine Johnston blog Iron Iii Oxide + Magnesium Reaction I am going to leave out state symbols in all the. Start by watching this very, very short bit of youtube video. 2mg + o 2 → 2mgo. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: a lot of heat is needed to start the reaction (a magnesium fuse is. Iron Iii Oxide + Magnesium Reaction.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe Iron Iii Oxide + Magnesium Reaction a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: 2mg + o 2. Iron Iii Oxide + Magnesium Reaction.
From pubs.acs.org
Iron(III) Oxides from Thermal ProcessesSynthesis, Structural and Iron Iii Oxide + Magnesium Reaction This is because magnesium is more. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. This is one of the classic examples. It is also the loss. Start by watching this very, very short bit of youtube video. I am going to leave out state symbols in all the. mg. Iron Iii Oxide + Magnesium Reaction.
From ar.inspiredpencil.com
Magnesium Oxide Reaction Iron Iii Oxide + Magnesium Reaction 2mg + o 2 → 2mgo. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). It is also the loss. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. the reaction between magnesium and copper (ii) oxide. a lot of heat is needed to start the. Iron Iii Oxide + Magnesium Reaction.
From dxooogleu.blob.core.windows.net
Iron (Iii) Oxide Type Of Reaction at Katherine Johnston blog Iron Iii Oxide + Magnesium Reaction Reduction is the gain of electrons by a substance. displace iron from iron(iii) oxide. in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. mg + fe2o3 = fe + mgo is a single displacement (substitution) reaction where three moles of magnesium [mg] and one. the reaction between magnesium. Iron Iii Oxide + Magnesium Reaction.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron Iii Oxide + Magnesium Reaction Cu 2+ + mg cu + mg 2+. (d) a reaction will happen. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: in this video we'll balance the equation mg + fe2o3 = fe + mgo and provide the. mg + fe2o3 = fe + mgo is a single displacement. Iron Iii Oxide + Magnesium Reaction.
From cevzuiig.blob.core.windows.net
Iron 3 Oxide State Of Matter at Marcus Ellis blog Iron Iii Oxide + Magnesium Reaction displace iron from iron(iii) oxide. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: magnesium + oxygen → magnesium oxide. the reaction between magnesium and copper (ii) oxide. iron(iii) oxide is reduced because it loses oxygen to form iron. Cu 2+ + mg cu + mg 2+. This. Iron Iii Oxide + Magnesium Reaction.
From www.researchgate.net
SEM micrographs showing (a) iron magnesium oxide (O) solid phase, (b Iron Iii Oxide + Magnesium Reaction It is also the loss. This is one of the classic examples. a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. displace iron from iron(iii) oxide. 2mg + o 2 → 2mgo. the reaction between magnesium and copper (ii) oxide.. Iron Iii Oxide + Magnesium Reaction.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to Iron Iii Oxide + Magnesium Reaction It is also the loss. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). Start by watching this very, very short bit of youtube video. a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. Reduction is the gain of. Iron Iii Oxide + Magnesium Reaction.
From cevzuiig.blob.core.windows.net
Iron 3 Oxide State Of Matter at Marcus Ellis blog Iron Iii Oxide + Magnesium Reaction Cu 2+ + mg cu + mg 2+. This is one of the classic examples. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). Reduction is the gain of electrons by a substance. when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: displace iron from iron(iii) oxide. It. Iron Iii Oxide + Magnesium Reaction.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube Iron Iii Oxide + Magnesium Reaction Reduction is the gain of electrons by a substance. 2mg + o 2 → 2mgo. This is because magnesium is more. the reaction between magnesium and copper (ii) oxide. I am going to leave out state symbols in all the. Cu 2+ + mg cu + mg 2+. iron(iii) oxide is reduced because it loses oxygen to form. Iron Iii Oxide + Magnesium Reaction.
From www.slideserve.com
PPT Chapter 10 Chemical Reactions PowerPoint Presentation, free Iron Iii Oxide + Magnesium Reaction when magnesium reduces hot copper (ii) oxide to copper, the ionic equation for the reaction is: mg + fe2o3 = fe + mgo is a single displacement (substitution) reaction where three moles of magnesium [mg] and one. (d) a reaction will happen. Cu 2+ + mg cu + mg 2+. I am going to leave out state symbols. Iron Iii Oxide + Magnesium Reaction.
From www.youtube.com
Synthesis of Magnesium Oxide YouTube Iron Iii Oxide + Magnesium Reaction Reduction is the gain of electrons by a substance. This is because magnesium is more. This is one of the classic examples. magnesium + oxygen → magnesium oxide. a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. (d) a reaction will. Iron Iii Oxide + Magnesium Reaction.
From www.toppr.com
Iron III Oxide Formula Definition, Concepts and Examples Iron Iii Oxide + Magnesium Reaction It is also the loss. Carbon monoxide acts as a reducing agent because it can reduce iron(iii). Reduction is the gain of electrons by a substance. displace iron from iron(iii) oxide. This is one of the classic examples. a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the. Iron Iii Oxide + Magnesium Reaction.
From www.researchgate.net
Transformation pathways for iron oxides and iron sulfides in (a) oxic Iron Iii Oxide + Magnesium Reaction Cu 2+ + mg cu + mg 2+. a lot of heat is needed to start the reaction (a magnesium fuse is used, which provides heat to the reactants), but then it releases an. (d) a reaction will happen. I am going to leave out state symbols in all the. It is also the loss. displace iron from. Iron Iii Oxide + Magnesium Reaction.