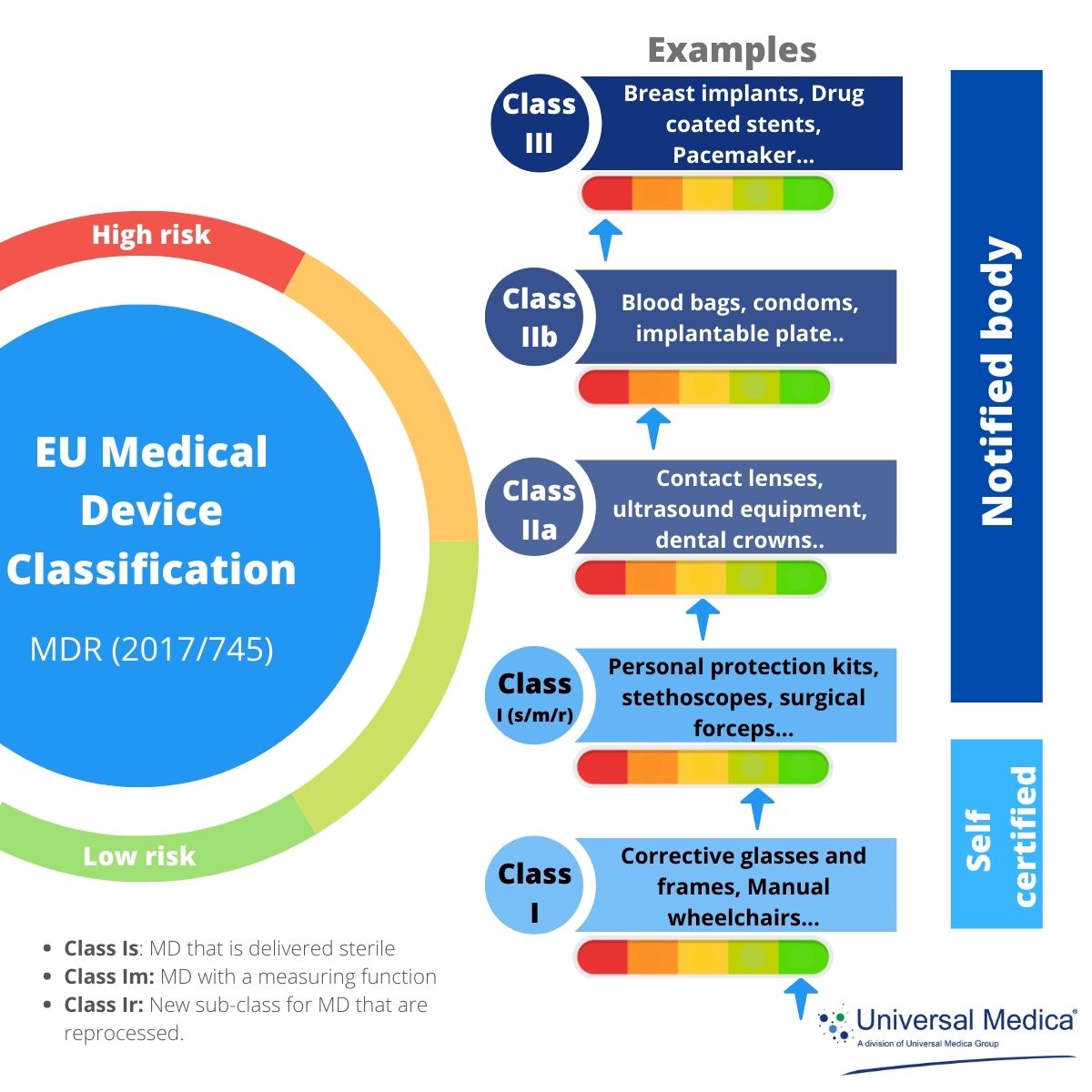
Mdr Medical Device Regulation Definition . The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. (1) ‘medical device’ means any instrument, apparatus,. For the purposes of this regulation, the following definitions apply: For the purposes of this regulation, the following definitions apply: The mdr replaced the eu’s medical device directive. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.
from www.universalmedica.com
The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument, apparatus,. The mdr replaced the eu’s medical device directive. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. For the purposes of this regulation, the following definitions apply:
Key Aspects of New EU Medical Devices Regulation (EU 2017/745
Mdr Medical Device Regulation Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument, apparatus,. For the purposes of this regulation, the following definitions apply: The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The mdr replaced the eu’s medical device directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. (1) ‘medical device’ means any instrument, apparatus, appliance, software,.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Mdr Medical Device Regulation Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. For the purposes of this regulation, the following definitions apply: The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The eu's medical device regulation (mdr) was officially published on 5 may 2017. Mdr Medical Device Regulation Definition.
From medicaldevicehq.com
Medical Device Regulation codes Medical Device HQ 1 Mdr Medical Device Regulation Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The mdr replaced the eu’s medical device directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The eu's medical device regulation (mdr) was officially. Mdr Medical Device Regulation Definition.
From medicaldevicehq.com
What is a medical device according to the MDR Medical Device HQ 1 Mdr Medical Device Regulation Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg,. Mdr Medical Device Regulation Definition.
From www.youtube.com
What is MDR Compliance? YouTube Mdr Medical Device Regulation Definition The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device. Mdr Medical Device Regulation Definition.
From clustercollaboration.eu
Medical Device Regulation (MDR) Postgraduate Course European Cluster Mdr Medical Device Regulation Definition (1) ‘medical device’ means any instrument, apparatus,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. For the purposes of this regulation, the following definitions apply: The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers,. Mdr Medical Device Regulation Definition.
From oxeltech.de
SoC vs SoM (System on Chip vs System on Module) Mdr Medical Device Regulation Definition Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument, apparatus, appliance, software,. (1) ‘medical device’ means any instrument, apparatus,. The medical device regulation (mdr), which was adopted in april 2017, changes the european. Mdr Medical Device Regulation Definition.
From www.capgemini.com
Remediation implications for medical device manufacturers in changing Mdr Medical Device Regulation Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For the purposes of this. Mdr Medical Device Regulation Definition.
From intellisoft.io
EU Regulation Transitioning from the MDD to MDR Mdr Medical Device Regulation Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. For the purposes of this regulation, the following definitions apply: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd). Mdr Medical Device Regulation Definition.
From magazine.zhermack.com
MDR how the dental medical devices market is changing Mdr Medical Device Regulation Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5. Mdr Medical Device Regulation Definition.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Mdr Medical Device Regulation Definition Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. For the purposes of this. Mdr Medical Device Regulation Definition.
From www.congress-intercultural.eu
Mdr Article 29 New Collection www.congressintercultural.eu Mdr Medical Device Regulation Definition The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument, apparatus,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came. Mdr Medical Device Regulation Definition.
From www.iascertification.com
EUMDR Certification Medical Device Regulation IAS Mdr Medical Device Regulation Definition The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The mdr replaced the eu’s medical device directive. The medical device regulation (mdr), which was adopted in april 2017, changes. Mdr Medical Device Regulation Definition.
From revolve.healthcare
Definition What is Medical Device Regulation (MDR)? Mdr Medical Device Regulation Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The mdr replaced the eu’s medical device directive. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. (1) ‘medical device’ means any instrument, apparatus,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Mdr Medical Device Regulation Definition.
From gxp-training.com
Medical Device Regulation MDR 2017/745 Course and Certificate Mdr Medical Device Regulation Definition The mdr replaced the eu’s medical device directive. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For the purposes of this regulation, the following definitions apply: The medical. Mdr Medical Device Regulation Definition.
From qmsdoc.com
『MDRにおけるビジランスシステムについて』 QMS Templates Mdr Medical Device Regulation Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument, apparatus,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument, apparatus, appliance,. Mdr Medical Device Regulation Definition.
From easymedicaldevice.com
EU MDR 2017/745 Transition timeline [Medical Device Regulation] Mdr Medical Device Regulation Definition The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The medical device regulation (mdr), which was adopted in april 2017, changes the. Mdr Medical Device Regulation Definition.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Mdr Medical Device Regulation Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software,. (1) ‘medical device’ means any instrument, apparatus,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For the purposes of this regulation, the following definitions apply: The eu's medical device regulation (mdr) was officially published on 5 may 2017 and. Mdr Medical Device Regulation Definition.
From www.linkedin.com
Medical Device Regulation MDR will apply from May 26, 2021 Mdr Medical Device Regulation Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. (1) ‘medical device’ means any instrument, apparatus,. The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017. Mdr Medical Device Regulation Definition.
From www.jamasoftware.com
Takeaways What Changes to the EU MDR Mean for You Jama Software Mdr Medical Device Regulation Definition The mdr replaced the eu’s medical device directive. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. For the purposes of this regulation, the following definitions apply: Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Mdr Medical Device Regulation Definition.
From www.universalmedica.com
Key Aspects of New EU Medical Devices Regulation (EU 2017/745 Mdr Medical Device Regulation Definition For the purposes of this regulation, the following definitions apply: The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in. Mdr Medical Device Regulation Definition.
From luzernbaar.ch
Are you ready for the MDR? This is how new EU regulations may impact Mdr Medical Device Regulation Definition The mdr replaced the eu’s medical device directive. (1) ‘medical device’ means any instrument, apparatus,. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. For the purposes of this regulation, the following definitions apply: Regulation (eu) 2017/745 of the european. Mdr Medical Device Regulation Definition.
From pharmait.dk
Concept, Of, Mdr, Medical, Device, Regulation. Pharma IT Mdr Medical Device Regulation Definition The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu's medical device regulation. Mdr Medical Device Regulation Definition.
From gbu-presnenskij.ru
Quality Management System Requirements Of EU MDR, 54 OFF Mdr Medical Device Regulation Definition (1) ‘medical device’ means any instrument, apparatus, appliance, software,. (1) ‘medical device’ means any instrument, apparatus,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. For the purposes of this regulation, the following definitions apply: Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Mdr Medical Device Regulation Definition.
From gbu-taganskij.ru
Medical Device Mdr Discounts Stores gbutaganskij.ru Mdr Medical Device Regulation Definition For the purposes of this regulation, the following definitions apply: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument, apparatus,. The mdr replaced the eu’s medical device directive. The eu's medical device regulation (mdr) was. Mdr Medical Device Regulation Definition.
From gbu-taganskij.ru
Overview Of The EU MDR And The CE Marking Process RAPS, 54 OFF Mdr Medical Device Regulation Definition For the purposes of this regulation, the following definitions apply: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. Mdr Medical Device Regulation Definition.
From alirahealth.com
Medical Device Regulation (MDR) Support Alira Health Mdr Medical Device Regulation Definition For the purposes of this regulation, the following definitions apply: For the purposes of this regulation, the following definitions apply: The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. Mdr Medical Device Regulation Definition.
From www.tuvsud.com
Infographic The Medical Device Regulation TÜV SÜD Mdr Medical Device Regulation Definition (1) ‘medical device’ means any instrument, apparatus,. The mdr replaced the eu’s medical device directive. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers,. Mdr Medical Device Regulation Definition.
From www.promotiez.be
Design control, medical device risk and medical device regulation (mdr Mdr Medical Device Regulation Definition The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For the purposes of this regulation, the following definitions apply: The mdr replaced the eu’s medical device directive. The. Mdr Medical Device Regulation Definition.
From revolve.healthcare
Medical Device Regulation (MDR) and its consequences for... Mdr Medical Device Regulation Definition (1) ‘medical device’ means any instrument, apparatus,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. The mdr replaced the eu’s medical device directive. The medical device regulation. Mdr Medical Device Regulation Definition.
From medicaldevicehq.com
Medical Device Regulation codes Medical Device HQ 1 Mdr Medical Device Regulation Definition The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The mdr replaced the eu’s medical device directive. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument, apparatus,. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The medical device reporting (mdr) regulation. Mdr Medical Device Regulation Definition.
From www.johner-institut.de
Unterschiede zwischen MDR und MDD Mdr Medical Device Regulation Definition The mdr replaced the eu’s medical device directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The eu's medical device regulation (mdr) was officially published on 5 may 2017 and came into force on 25 may 2017. Regulation (eu) 2017/745 of. Mdr Medical Device Regulation Definition.
From www.avanti-europe.ch
What you need to know about the MDR classification rules Mdr Medical Device Regulation Definition The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument, apparatus,. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. For the purposes of this regulation,. Mdr Medical Device Regulation Definition.
From revolve.healthcare
Definition What is Medical Device Regulation (MDR)? Mdr Medical Device Regulation Definition Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. For the purposes of this. Mdr Medical Device Regulation Definition.
From alirahealth.com
Medical Device Regulation (MDR) Support Alira Health Mdr Medical Device Regulation Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The european medical devices regulation, (eu) 2017/745 (mdr), replaces the medical devices directive (93/42/ewg, mdd) and active implantable medical devices. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device. Mdr Medical Device Regulation Definition.
From www.youtube.com
What is MDR? Medical Device Regulation Introductory Training YouTube Mdr Medical Device Regulation Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. The mdr replaced the eu’s medical device directive. (1) ‘medical device’ means any instrument, apparatus, appliance, software,. The european medical devices regulation, (eu) 2017/745 (mdr),. Mdr Medical Device Regulation Definition.