Bromine Hydrogen Bromide Boiling Point . Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Cox, wagman, et al., 1984: Aqueous solutions that are 47.38% hbr by. Chemspider record containing structure, synonyms, properties, vendors and database links for. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Quantity value units method reference comment; Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. A solution of the gas in water is.
from www.numerade.com
A solution of the gas in water is. Cox, wagman, et al., 1984: Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Chemspider record containing structure, synonyms, properties, vendors and database links for. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Quantity value units method reference comment; Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Aqueous solutions that are 47.38% hbr by.
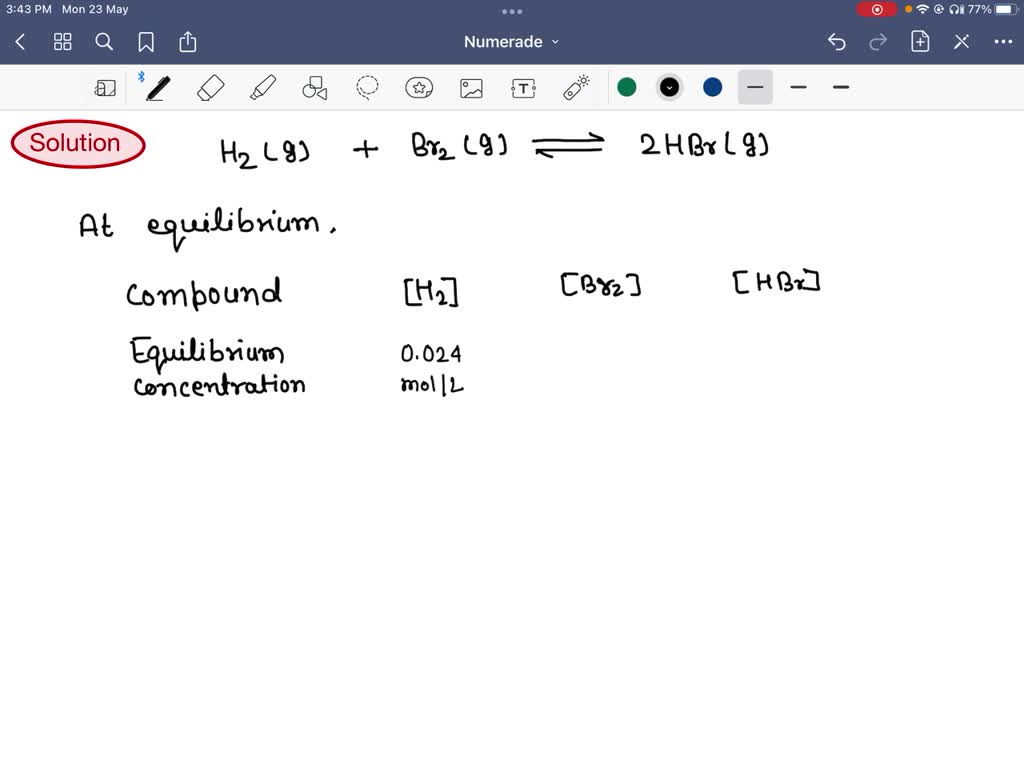
SOLVED Question 14 (1 point) Hydrogen is the lightest of elements with
Bromine Hydrogen Bromide Boiling Point Cox, wagman, et al., 1984: Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Quantity value units method reference comment; Cox, wagman, et al., 1984: Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. A solution of the gas in water is. Chemspider record containing structure, synonyms, properties, vendors and database links for. Aqueous solutions that are 47.38% hbr by.
From www.gauthmath.com
What mass of reactants are used during the reaction? Hydrogen + Bromine Bromine Hydrogen Bromide Boiling Point Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Cox, wagman, et al., 1984: Aqueous solutions that are 47.38% hbr by. A solution of the gas in water is. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its. Bromine Hydrogen Bromide Boiling Point.
From www.chegg.com
Solved The normal boiling point (at P=1 bar) of bromine Bromine Hydrogen Bromide Boiling Point Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Chemspider record containing structure, synonyms, properties, vendors and database links for. Aqueous solutions that are 47.38% hbr by. Cox, wagman, et al., 1984: Quantity value units method reference comment;. Bromine Hydrogen Bromide Boiling Point.
From favpng.com
Hydrogen Bromide Hydrobromic Acid Bromine, PNG, 1100x1043px, Hydrogen Bromine Hydrogen Bromide Boiling Point A solution of the gas in water is. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Chemspider record containing structure, synonyms, properties, vendors and database links for. Aqueous solutions that are 47.38% hbr by. Hydrogen bromide (along. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVED Question 14 (1 point) Hydrogen is the lightest of elements with Bromine Hydrogen Bromide Boiling Point Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Chemspider record containing structure, synonyms, properties, vendors and database links for. A solution of the gas in water is. Quantity value units method reference comment; Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVED For the reaction between hydrogen and bromine which produces Bromine Hydrogen Bromide Boiling Point Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. A solution of the gas in. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVED Question 14 (1 point) Hydrogen is the lightest of elements with Bromine Hydrogen Bromide Boiling Point Cox, wagman, et al., 1984: Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Chemspider record containing structure, synonyms, properties, vendors and database links for. A. Bromine Hydrogen Bromide Boiling Point.
From www.shutterstock.com
Bromine Parodic Table Element Boiling Melting Stock Vector (Royalty Bromine Hydrogen Bromide Boiling Point Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Quantity value units method reference comment; Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is.. Bromine Hydrogen Bromide Boiling Point.
From www.istockphoto.com
Vector Ballandstick Icon Of Hydrobromic Acid Or Hydrogen Bromide Hbr Bromine Hydrogen Bromide Boiling Point Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Quantity value units method reference comment;. Bromine Hydrogen Bromide Boiling Point.
From wisc.pb.unizin.org
M10Q2 Melting and Boiling Point Comparisons Chem 103/104 Resource Book Bromine Hydrogen Bromide Boiling Point Aqueous solutions that are 47.38% hbr by. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. A solution of the gas in water is. Cox, wagman, et al., 1984: Chemspider record containing structure, synonyms, properties, vendors and database links for. Hydrogen bromide (along with hydrobromic acid). Bromine Hydrogen Bromide Boiling Point.
From www.gauthmath.com
during the reaction? Hydrogen + Bromine Hydrogen Bromide 16.25 g Give Bromine Hydrogen Bromide Boiling Point Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Cox, wagman, et al., 1984: Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Chemspider record containing structure, synonyms, properties, vendors and database links for. A. Bromine Hydrogen Bromide Boiling Point.
From socratic.org
The liquefied hydrogen halides have the normal boiling points given Bromine Hydrogen Bromide Boiling Point Chemspider record containing structure, synonyms, properties, vendors and database links for. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Cox, wagman, et al., 1984: Aqueous solutions that are 47.38% hbr by. Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at. Bromine Hydrogen Bromide Boiling Point.
From www.semanticscholar.org
Figure 11 from Production of hydrogen bromide by brominemethane Bromine Hydrogen Bromide Boiling Point Chemspider record containing structure, synonyms, properties, vendors and database links for. Cox, wagman, et al., 1984: Aqueous solutions that are 47.38% hbr by. A solution of the gas in water is. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric. Bromine Hydrogen Bromide Boiling Point.
From www.gauthmath.com
What mass of reactants are used during the reaction? Hydrogen + Bromine Bromine Hydrogen Bromide Boiling Point A solution of the gas in water is. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Aqueous solutions that are 47.38% hbr by. Cox, wagman,. Bromine Hydrogen Bromide Boiling Point.
From www.nuclear-power.com
Bromine Melting Point Boiling Point Bromine Hydrogen Bromide Boiling Point Aqueous solutions that are 47.38% hbr by. Chemspider record containing structure, synonyms, properties, vendors and database links for. Cox, wagman, et al., 1984: Quantity value units method reference comment; Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is.. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVED The normal boiling point of bromine is 58.8 °C. Using the heat Bromine Hydrogen Bromide Boiling Point Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. A solution of the gas in water is. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Cox,. Bromine Hydrogen Bromide Boiling Point.
From www.bartleby.com
Answered Hydrogen bromide is a compound of the… bartleby Bromine Hydrogen Bromide Boiling Point Aqueous solutions that are 47.38% hbr by. Quantity value units method reference comment; Chemspider record containing structure, synonyms, properties, vendors and database links for. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine. Bromine Hydrogen Bromide Boiling Point.
From www.slideshare.net
Bromine berry Bromine Hydrogen Bromide Boiling Point Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. A solution of the gas in. Bromine Hydrogen Bromide Boiling Point.
From imgbin.com
Hydrogen Bromide Hydrogen Fluoride Hydrochloric Acid Boiling Point PNG Bromine Hydrogen Bromide Boiling Point Cox, wagman, et al., 1984: Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. A solution of the gas in water is. Chemspider record containing structure, synonyms, properties, vendors and database links for. Hydrogen bromide (along with hydrobromic. Bromine Hydrogen Bromide Boiling Point.
From www.semanticscholar.org
Figure 1 from Production of hydrogen bromide by brominemethane Bromine Hydrogen Bromide Boiling Point Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Quantity value units method reference comment; Cox, wagman, et. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVED Give structures and names of the products, if any, expected Bromine Hydrogen Bromide Boiling Point A solution of the gas in water is. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Chemspider record containing structure, synonyms, properties, vendors and database links for. Aqueous solutions that are 47.38% hbr by. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work. Bromine Hydrogen Bromide Boiling Point.
From www.chegg.com
Solved Bromine and water react to form hydrogen bromide and Bromine Hydrogen Bromide Boiling Point Quantity value units method reference comment; Aqueous solutions that are 47.38% hbr by. Cox, wagman, et al., 1984: Chemspider record containing structure, synonyms, properties, vendors and database links for. A solution of the gas in water is. Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Unlike hydrogen fluoride,. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVED Bromine has a boiling point of 59 *C. What is the correct Bromine Hydrogen Bromide Boiling Point Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Aqueous solutions that are 47.38% hbr by. A solution of the gas in water is. Quantity value. Bromine Hydrogen Bromide Boiling Point.
From www.chegg.com
Solved The formation of Hydrogen Bromide from hydrogen and Bromine Hydrogen Bromide Boiling Point Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Cox, wagman, et al., 1984: Chemspider record containing structure, synonyms, properties, vendors and database links for. A solution of the gas in water is. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVEDHydrogen bromide into hydrogen gas and bromine gas Bromine Hydrogen Bromide Boiling Point Chemspider record containing structure, synonyms, properties, vendors and database links for. Aqueous solutions that are 47.38% hbr by. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Cox, wagman, et al., 1984: Hydrogen bromide (along with hydrobromic acid). Bromine Hydrogen Bromide Boiling Point.
From andreakruwwoodward.blogspot.com
The Boiling Points of Hydrogen Chloride and Hydrogen Bromide Bromine Hydrogen Bromide Boiling Point A solution of the gas in water is. Aqueous solutions that are 47.38% hbr by. Cox, wagman, et al., 1984: Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Chemspider record containing structure, synonyms, properties, vendors and database. Bromine Hydrogen Bromide Boiling Point.
From www.britannica.com
bromine Properties, Uses, & Facts Britannica Bromine Hydrogen Bromide Boiling Point Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Cox, wagman, et al., 1984: Chemspider record containing structure, synonyms, properties, vendors and database links for. A solution of the gas in water is. Quantity value units method reference. Bromine Hydrogen Bromide Boiling Point.
From www.alamy.com
Hydrogen bromide (HBr) molecule. Skeletal formula Stock Vector Image Bromine Hydrogen Bromide Boiling Point Cox, wagman, et al., 1984: Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Quantity value units method reference comment; Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVEDHydrogen burns in an atmosphere of bromine to give hydrogen Bromine Hydrogen Bromide Boiling Point Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Aqueous solutions that are 47.38% hbr by. Cox, wagman, et al., 1984: Chemspider record containing structure, synonyms, properties, vendors and database links for. Quantity value units method reference comment; Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVED The reaction below shows the formation of hydrogen bromide gas Bromine Hydrogen Bromide Boiling Point Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Aqueous solutions that are 47.38% hbr by. Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Chemspider record containing structure, synonyms, properties, vendors and database links. Bromine Hydrogen Bromide Boiling Point.
From byjus.com
Write the molecular formulae for the compound Copper II Bromide Bromine Hydrogen Bromide Boiling Point Cox, wagman, et al., 1984: A solution of the gas in water is. Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Aqueous solutions that are 47.38% hbr by. Quantity value units method reference comment; Chemspider record containing. Bromine Hydrogen Bromide Boiling Point.
From www.slideserve.com
PPT Lesson 1 Introduction to Redox Reactions PowerPoint Presentation Bromine Hydrogen Bromide Boiling Point Quantity value units method reference comment; Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is. Aqueous solutions that are 47.38% hbr by. Cox, wagman, et al., 1984: Other bromine compounds of significance include hydrogen bromide (hbr), a colorless. Bromine Hydrogen Bromide Boiling Point.
From giowmcmfq.blob.core.windows.net
Bromine Br Boiling Point at Dennis blog Bromine Hydrogen Bromide Boiling Point Quantity value units method reference comment; Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Cox, wagman, et al., 1984: A solution of the gas in water is. Aqueous solutions that are 47.38% hbr by. Chemspider record containing structure, synonyms, properties, vendors and database links for.. Bromine Hydrogen Bromide Boiling Point.
From www.gauthmath.com
Solved If you wanted to change the polarity of hydrogen bromide (HBr Bromine Hydrogen Bromide Boiling Point Chemspider record containing structure, synonyms, properties, vendors and database links for. Aqueous solutions that are 47.38% hbr by. Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. A solution of the gas in water is. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as. Bromine Hydrogen Bromide Boiling Point.
From material-properties.org
Bromine Thermal Properties Melting Point Thermal Conductivity Bromine Hydrogen Bromide Boiling Point Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °c. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Cox, wagman, et al., 1984: Chemspider record containing structure, synonyms, properties, vendors and database links for. Unlike. Bromine Hydrogen Bromide Boiling Point.
From www.numerade.com
SOLVED Which acts as an electrophile in the following reaction? FeCl3 Bromine Hydrogen Bromide Boiling Point Aqueous solutions that are 47.38% hbr by. A solution of the gas in water is. Other bromine compounds of significance include hydrogen bromide (hbr), a colorless gas used as a reducing agent and a catalyst in organic reactions. Cox, wagman, et al., 1984: Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200. Bromine Hydrogen Bromide Boiling Point.