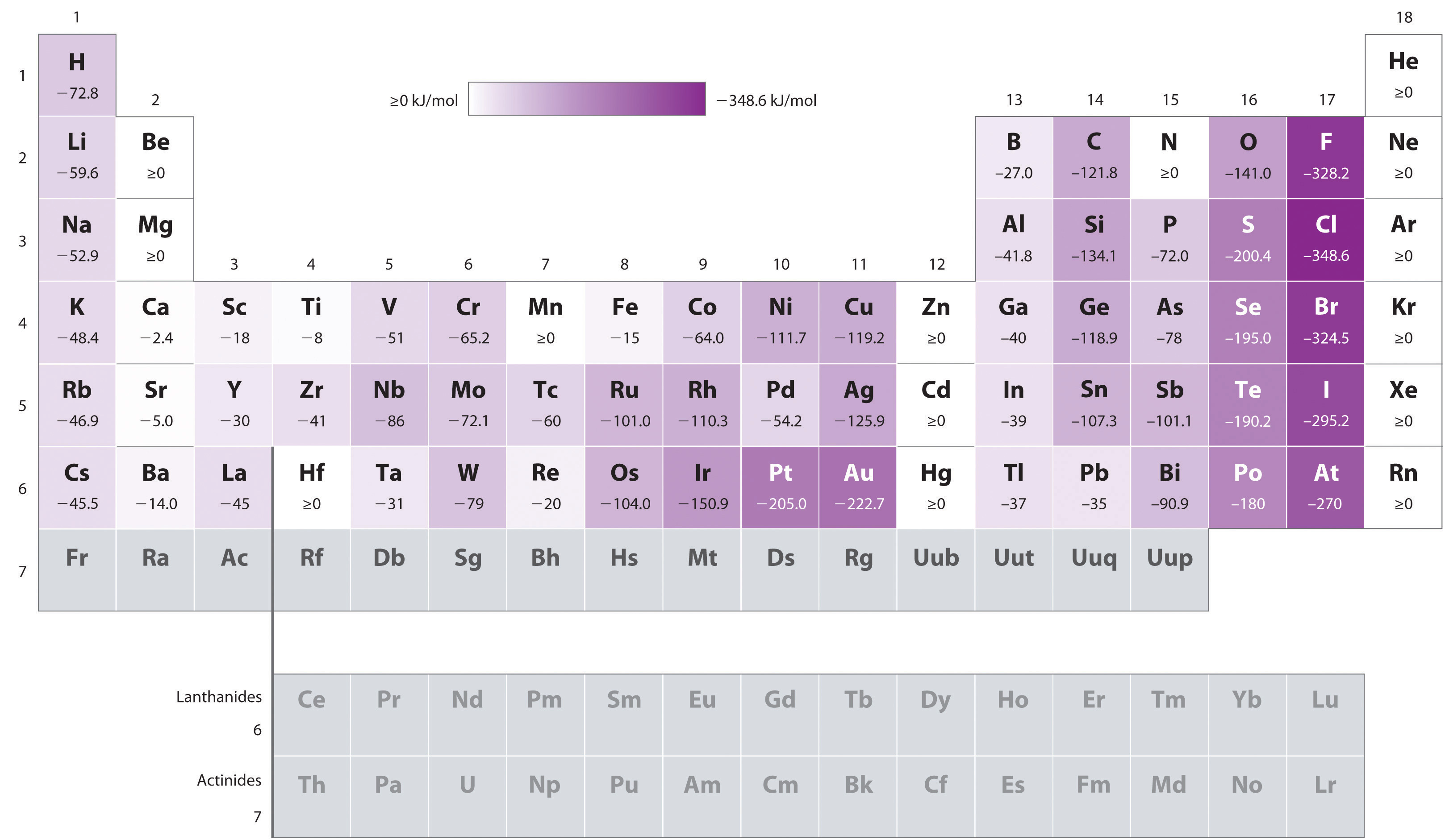
Cl Electron Affinity . the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. — electron affinity of chlorine is 349 kj/mol. — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. In general, elements with the most negative electron affinities.
from chem.libretexts.org
the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In general, elements with the most negative electron affinities. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. In chemistry and atomic physics, the electron affinity of an atom or. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. — electron affinity of chlorine is 349 kj/mol.
1.1.2.4 Electron Affinity Chemistry LibreTexts
Cl Electron Affinity — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. — electron affinity of chlorine is 349 kj/mol. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In chemistry and atomic physics, the electron affinity of an atom or. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. In general, elements with the most negative electron affinities. — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Cl Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. the electron affinity (ea) of an element. Cl Electron Affinity.
From www.numerade.com
SOLVED Use this electron affinity, EA, data for Cl, Br, and I (and Cl Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. In general, elements with the most negative electron affinities. 13 rows — the electron affinity is a measure of the. Cl Electron Affinity.
From www.numerade.com
SOLVEDUse data in this chapter to determine the following. a. the Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. — electron affinity of chlorine. Cl Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Cl Electron Affinity — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. In general, elements with the most negative. Cl Electron Affinity.
From circuitwiringgena.z13.web.core.windows.net
Atomic Orbital Diagram For Chlorine Cl Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. In chemistry and atomic physics, the electron affinity. Cl Electron Affinity.
From www.youtube.com
Chlorine has ___________ electron affinity than fluorine. YouTube Cl Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. 13 rows — the electron affinity is. Cl Electron Affinity.
From chem.libretexts.org
1.1.2.4 Electron Affinity Chemistry LibreTexts Cl Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. the electron affinity (ea) of. Cl Electron Affinity.
From www.pinterest.com
Periodic Table Electronegativity Trend Ionization energy Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. — electron affinity of chlorine is 349 kj/mol. — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Cl Electron Affinity.
From www.youtube.com
3.2 Electron affinity (SL) YouTube Cl Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. the electron affinity (ea) of. Cl Electron Affinity.
From www.breakingatom.com
Electron Affinity of The Elements Cl Electron Affinity the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. In chemistry and atomic physics, the. Cl Electron Affinity.
From study.com
Electron Affinity Definition, Trends & Examples Lesson Cl Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. — electron affinity is the. Cl Electron Affinity.
From www.angelo.edu
The Parts of the Periodic Table Cl Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. In general, elements with the most negative electron affinities. — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Cl Electron Affinity.
From www.numerade.com
SOLVEDUsing data from the text, determine the following values Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. — electron affinity of chlorine is 349. Cl Electron Affinity.
From www.youtube.com
3.5CElectron Affinity YouTube Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. — electron affinity of chlorine is 349. Cl Electron Affinity.
From www.toppr.com
The electron affinity of the following elements can be arranged as Cl Cl Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. — electron affinity of chlorine is 349 kj/mol. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to. Cl Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Cl Electron Affinity electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. the electron affinity (ea) of an element. Cl Electron Affinity.
From eduinput.com
Electron Affinity, definition, examples, significance, factors Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. the electron affinity (ea) of. Cl Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. In chemistry and atomic physics, the electron affinity of an atom or. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a. Cl Electron Affinity.
From university.pressbooks.pub
(Libre Clone) 4.4 Ionization energy and Electron Affinity Chemistry Cl Electron Affinity — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. 13 rows — the electron affinity is a. Cl Electron Affinity.
From apomillionaire.weebly.com
Electron affinity chart apomillionaire Cl Electron Affinity — electron affinity of chlorine is 349 kj/mol. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Cl Electron Affinity.
From www.pearson.com
The periodic table with electron affinity values is shown below Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. the electron affinity (ea) of. Cl Electron Affinity.
From www.pinterest.com
Electron Affinity Trend and Definition Electron affinity, Electrons Cl Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. In general, elements with the most negative electron affinities. — electron affinity of chlorine is 349 kj/mol. — electron affinity is the amount of energy change (δe) that occurs when an. Cl Electron Affinity.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. In general, elements with the most negative electron affinities. — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Cl Electron Affinity.
From www.youtube.com
Electron Affinity Part2 Chemistry General Concepts YouTube Cl Electron Affinity the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. — electron affinity of chlorine is 349 kj/mol. — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Cl Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Cl Electron Affinity — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. In general, elements with the most negative electron affinities. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added.. Cl Electron Affinity.
From www.sliderbase.com
Periodic Behavior Presentation Chemistry Cl Electron Affinity — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. In chemistry and atomic physics, the electron affinity of an atom or. — electron affinity of chlorine is 349 kj/mol. the electron affinity (ea) of an element is the energy change that. Cl Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most. Cl Electron Affinity.
From www.youtube.com
Electron Affinity Equations YouTube Cl Electron Affinity — electron affinity of chlorine is 349 kj/mol. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to. Cl Electron Affinity.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Cl Electron Affinity — electron affinity of chlorine is 349 kj/mol. — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a. Cl Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Cl Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. In general, elements with the most negative electron affinities. the electron affinity is the potential energy change of the atom. Cl Electron Affinity.
From periodictableguide.com
Electron Affinity Chart (Labeled Periodic table + List) Cl Electron Affinity 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity. Cl Electron Affinity.
From www.youtube.com
Electron Affinity Trends and Some Irregularity YouTube Cl Electron Affinity — electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. — electron affinity of chlorine is 349 kj/mol.. Cl Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Cl Electron Affinity — electron affinity of chlorine is 349 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. — electron affinity is the amount of energy change (δe) that. Cl Electron Affinity.
From www.youtube.com
know Electron affinity in just 4 minutes..why E.A of cl is greater than Cl Electron Affinity — electron affinity of chlorine is 349 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or. 13 rows — the electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative. the electron affinity (ea) of an element is the energy change. Cl Electron Affinity.
From www.savemyexams.com
Electron Affinity & Trends of Group 16 & 17 Elements CIE A Level Cl Electron Affinity the electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. — electron affinity of chlorine is 349 kj/mol. In general, elements with the most negative electron affinities. In chemistry and atomic physics, the electron affinity of an atom or. — electron. Cl Electron Affinity.