Molar Heat Capacity Of Nitrogen At Constant Pressure . follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] Density and specific weight ; heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas.
from www.chegg.com
the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. Density and specific weight ; Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\]
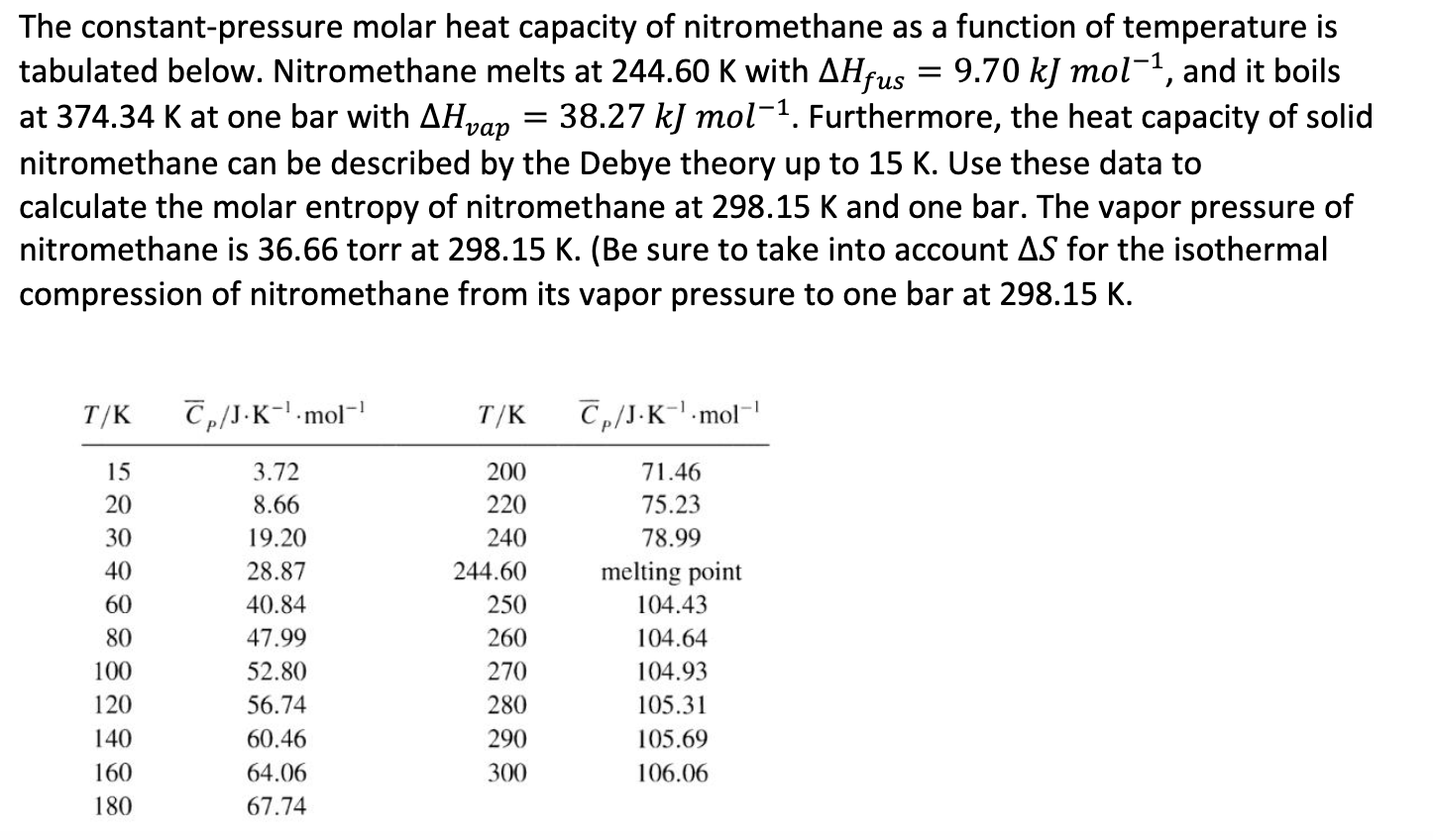
Solved The constantpressure molar heat capacity of
Molar Heat Capacity Of Nitrogen At Constant Pressure follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : Density and specific weight ; that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature :
From www.numerade.com
SOLVED Compute the specific heat capacity at constant volume of Molar Heat Capacity Of Nitrogen At Constant Pressure in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Furthermore, since the ideal gas expands. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.semanticscholar.org
Table 1 from Molar Heat Capacity (Cv) for Saturated and Compressed Molar Heat Capacity Of Nitrogen At Constant Pressure in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Furthermore, since the ideal gas expands. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.researchgate.net
VARIATION OF SPECIFIC HEAT CAPACITY AT CONSTANT PRESSURE (CP) AS A Molar Heat Capacity Of Nitrogen At Constant Pressure heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. Furthermore, since the ideal gas expands. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.coursehero.com
[Solved] Find the specific heat at constant pressure of nitrogen gas Molar Heat Capacity Of Nitrogen At Constant Pressure Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. follow the links below to get values for the listed properties of nitrogen at. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From dxoqmmcyh.blob.core.windows.net
Heat Capacity Ratio Of Nitrogen Gas at William Parker blog Molar Heat Capacity Of Nitrogen At Constant Pressure Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. follow the links below to. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From dxonmopmx.blob.core.windows.net
Specific Heat Capacity Of Nitrogen Gas At Constant Pressure at Jessica Molar Heat Capacity Of Nitrogen At Constant Pressure Density and specific weight ; that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. follow the links below to get values for the. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.chegg.com
Solved (6)The constantpressure molar heat capacity of Molar Heat Capacity Of Nitrogen At Constant Pressure in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] heat capacity at constant pressure. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.researchgate.net
The molar heat capacity at constant pressure (C P ° ,m ) vs Molar Heat Capacity Of Nitrogen At Constant Pressure heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] follow the links below to get values for the listed properties of nitrogen at varying pressure and. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.coursehero.com
[Solved] The constant pressure molar heat capacity of nitrogen is given Molar Heat Capacity Of Nitrogen At Constant Pressure the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] follow the links below to get values for the listed properties of nitrogen at varying pressure and. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Molar Heat Capacity Of Nitrogen At Constant Pressure in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. Density and specific weight ; heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. . Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.youtube.com
Calculate the average molar heat capacity at constant volume of a Molar Heat Capacity Of Nitrogen At Constant Pressure Density and specific weight ; that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From byjus.com
why is molar heat capacity at cons†an t pressure always greater than Molar Heat Capacity Of Nitrogen At Constant Pressure heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] that is, when enough heat is added to increase the temperature of one mole of ideal gas. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.chegg.com
Solved Part A Compute the specific heat capacity at constant Molar Heat Capacity Of Nitrogen At Constant Pressure the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : Density and specific weight ; heat capacity at constant pressure (liquid) as a function of temperature and pressure. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.chegg.com
Solved The constantpressure molar heat capacity of Molar Heat Capacity Of Nitrogen At Constant Pressure follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. Density and specific weight ; the. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation Molar Heat Capacity Of Nitrogen At Constant Pressure Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. in this case, the heat is added at constant pressure, and we write \. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From dxonmopmx.blob.core.windows.net
Specific Heat Capacity Of Nitrogen Gas At Constant Pressure at Jessica Molar Heat Capacity Of Nitrogen At Constant Pressure in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. Density and specific weight ; that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.toppr.com
The Molar heat capacities of nitrogen at constant pressure and constant Molar Heat Capacity Of Nitrogen At Constant Pressure heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. in this case, the heat is added at constant pressure, and we write \. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.chegg.com
Solved The difference between heat capacity at constant Molar Heat Capacity Of Nitrogen At Constant Pressure Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. the specific heat (= specific. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.researchgate.net
Behavior of the molar heat capacity at constant pressure (50 atm Molar Heat Capacity Of Nitrogen At Constant Pressure follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\]. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From dxonmopmx.blob.core.windows.net
Specific Heat Capacity Of Nitrogen Gas At Constant Pressure at Jessica Molar Heat Capacity Of Nitrogen At Constant Pressure in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. Density and specific weight ; that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.youtube.com
Molar Heat Capacity Problems Physics YouTube Molar Heat Capacity Of Nitrogen At Constant Pressure Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.toppr.com
The temperature of 3 kg of nitrogen is raised from 10 ^o C to 100 ^o C Molar Heat Capacity Of Nitrogen At Constant Pressure heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. Density and specific weight ; that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. Furthermore, since the ideal gas expands against a constant pressure,. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.youtube.com
Molar Specific Heat for Constant Volume and Constant Pressure YouTube Molar Heat Capacity Of Nitrogen At Constant Pressure the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. Density and specific weight ; Furthermore,. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.numerade.com
SOLVED A) Compute the specific heat capacity at constant volume of Molar Heat Capacity Of Nitrogen At Constant Pressure Density and specific weight ; follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. Furthermore, since the ideal gas expands against a constant pressure, \. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.researchgate.net
Molar specific heat capacity of solid chemical elements at constant Molar Heat Capacity Of Nitrogen At Constant Pressure Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.youtube.com
Heat Capacity at Constant Volume Derivation Explanation YouTube Molar Heat Capacity Of Nitrogen At Constant Pressure the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. Density and specific weight ; heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. that is, when enough heat is added to increase the temperature of. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From kunduz.com
[ANSWERED] An ideal gas has molar heat capacity at constant pressure Cp Molar Heat Capacity Of Nitrogen At Constant Pressure Density and specific weight ; that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] the specific heat (= specific heat capacity) at constant. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From askfilo.com
The molar heat capacity of water at constant pressure, C, is 75 J K−1 mol.. Molar Heat Capacity Of Nitrogen At Constant Pressure that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. in this case, the heat is added at constant pressure, and we write \ [dq = c_ {p}ndt,\] where \ (c_p\) is the molar heat capacity at constant pressure of the gas. heat capacity. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.numerade.com
SOLVED The constant pressure molar heat capacity, Cp.m., of nitrogen Molar Heat Capacity Of Nitrogen At Constant Pressure that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. Density and specific weight ; heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. follow the links below to get values for the. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.numerade.com
SOLVED Assume the constant pressure heat capacity of nitrogen gas is Molar Heat Capacity Of Nitrogen At Constant Pressure Density and specific weight ; heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. in this case, the heat is added at constant pressure, and we. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.chegg.com
Solved 9. The molar heat capacity of nitrogen at 1 bar is Molar Heat Capacity Of Nitrogen At Constant Pressure Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] Density and specific weight ; heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. that is, when enough heat is added to increase the temperature of. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.semanticscholar.org
Figure 7 from Molar Heat Capacity (Cv) for Saturated and Compressed Molar Heat Capacity Of Nitrogen At Constant Pressure Density and specific weight ; follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature from 63.151 k to 126.192 k. the specific heat (= specific heat capacity) at constant pressure and constant volume. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.bartleby.com
Molar Specific Heat bartleby Molar Heat Capacity Of Nitrogen At Constant Pressure Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. heat capacity at constant pressure (liquid) as a function of temperature and pressure temperature. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From askfilo.com
182 Thermodynamics 17. The molar heat capacity at constant pressure of an.. Molar Heat Capacity Of Nitrogen At Constant Pressure that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. follow the links below to get values for the listed properties of nitrogen at varying pressure and temperature : Density and specific weight ; heat capacity at constant pressure (liquid) as a function of. Molar Heat Capacity Of Nitrogen At Constant Pressure.
From www.chegg.com
The constantpressure molar heat capacity of Molar Heat Capacity Of Nitrogen At Constant Pressure that is, when enough heat is added to increase the temperature of one mole of ideal gas by one degree kelvin at constant. Furthermore, since the ideal gas expands against a constant pressure, \ [d (pv) = d (rnt)\] becomes \ [pdv = rndt.\] the specific heat (= specific heat capacity) at constant pressure and constant volume processes,. Molar Heat Capacity Of Nitrogen At Constant Pressure.