Surface Area Of Liquid . The surface area is the amount of that liquid that is. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. This applies to reactions involving a solid and a gas, or a solid and a. However, the molecules on the. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The stronger the intermolecular interactions, the greater the surface tension. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. Surface body formulas and calculation. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is involved in.
from variasclasses.blogspot.com
Surface tension is the energy required to increase the surface area of a liquid by a given amount. However, the molecules on the. This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is involved in. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. Surface body formulas and calculation. This applies to reactions involving a solid and a gas, or a solid and a. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The surface area is the amount of that liquid that is.
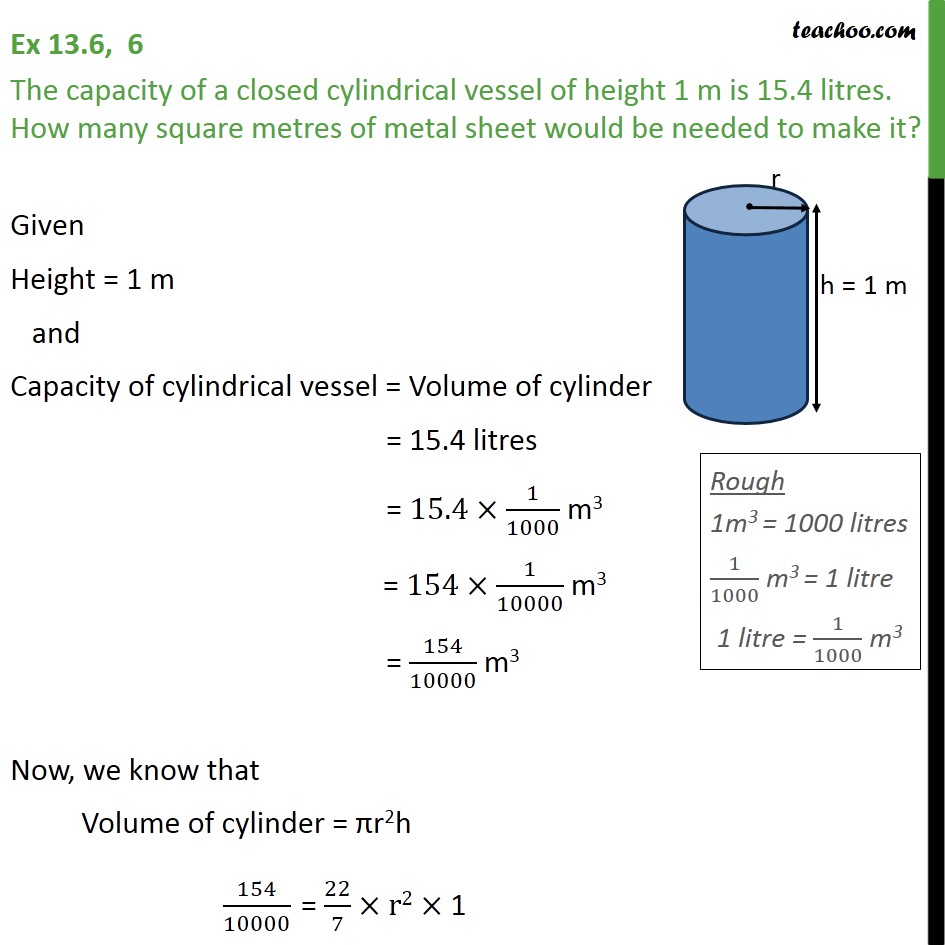
Ncert Solutions For Class 9 Maths Chapter 13 Exercise 136 Várias Classes
Surface Area Of Liquid The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. This applies to reactions involving a solid and a gas, or a solid and a. Surface body formulas and calculation. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is involved in. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the greater the surface tension. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. The surface area is the amount of that liquid that is. However, the molecules on the. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces.
From variasclasses.blogspot.com
Ncert Solutions For Class 9 Maths Chapter 13 Exercise 136 Várias Classes Surface Area Of Liquid Surface body formulas and calculation. This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is involved in. The stronger the intermolecular interactions, the greater the surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surfactants are. Surface Area Of Liquid.
From www.toppr.com
The surface tension of water at 20^∘C is 73 dynes cm^1 . The minimum Surface Area Of Liquid Surface tension is the energy required to increase the surface area of a liquid by a given amount. This applies to reactions involving a solid and a gas, or a solid and a. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. Surface tension is. Surface Area Of Liquid.
From www.researchgate.net
Temporal changes of surface area of water bodies Download Scientific Surface Area Of Liquid Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is involved in. However, the molecules on the. This applies to reactions involving a solid and a gas, or a. Surface Area Of Liquid.
From bottlefirst.com
Surface Area Of A Plastic Water Bottle StepbyStep Guide! Surface Area Of Liquid The surface area is the amount of that liquid that is. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. This applies to reactions involving a solid and a gas, or a solid and a. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions. Surface Area Of Liquid.
From www.researchgate.net
(a) Water depthVolume relationship and (b) water surface areavolume Surface Area Of Liquid The stronger the intermolecular interactions, the greater the surface tension. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. Surface tension is the energy. Surface Area Of Liquid.
From www.slideserve.com
PPT The surface phenomenon of liquid PowerPoint Presentation, free Surface Area Of Liquid The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. This page describes and explains the effect of changing the surface area of a solid. Surface Area Of Liquid.
From onlineengineeringnotes.com
Hydrostatic Forces on different submerged surface in liquid Surface Area Of Liquid Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. The surface area is the amount of that liquid that is. The. Surface Area Of Liquid.
From www.logiota.com
Surface Tension States of Matter Physical Chemistry Chemistry Surface Area Of Liquid The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. This applies to reactions involving a solid and a gas, or a solid and a. However, the molecules on the. Surface body formulas and calculation. This page describes and explains the effect of changing the surface. Surface Area Of Liquid.
From www.britannica.com
Surface water hydrology Britannica Surface Area Of Liquid The surface area is the amount of that liquid that is. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. However,. Surface Area Of Liquid.
From physicsexperiments.eu
Dependence of Evaporation Rate of Liquid on Liquid Surface Area Surface Area Of Liquid This applies to reactions involving a solid and a gas, or a solid and a. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the greater the surface. Surface Area Of Liquid.
From www.slideserve.com
PPT The surface phenomenon of liquid PowerPoint Presentation, free Surface Area Of Liquid However, the molecules on the. Surface body formulas and calculation. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is. Surface Area Of Liquid.
From www.slideshare.net
Evaporation Surface Area Of Liquid The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. However, the molecules on the. The stronger the intermolecular interactions, the greater the surface tension. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length. Surface Area Of Liquid.
From learningkurugon1.z22.web.core.windows.net
Gcse Maths Surface Area Of A Sphere Surface Area Of Liquid The surface area is the amount of that liquid that is. This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is involved in. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Surface body formulas and calculation. The molecules. Surface Area Of Liquid.
From www.researchgate.net
The ratio of the free surface area of liquid before and after the Surface Area Of Liquid The surface area is the amount of that liquid that is. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. Surface body formulas and calculation. The stronger the intermolecular interactions, the greater the surface tension. However, the molecules on the. This page describes and explains. Surface Area Of Liquid.
From mavink.com
Evaporation Surface Area Surface Area Of Liquid This applies to reactions involving a solid and a gas, or a solid and a. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. Surface tension is defined as the energy. Surface Area Of Liquid.
From www.chegg.com
Solved Determine height of water, h, in the glass if the Surface Area Of Liquid The surface area is the amount of that liquid that is. This applies to reactions involving a solid and a gas, or a solid and a. However, the molecules on the. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. This page describes and explains the effect of changing the surface area. Surface Area Of Liquid.
From www.researchgate.net
The surface area of liquid spreading over plastic with a polymer mesh Surface Area Of Liquid This applies to reactions involving a solid and a gas, or a solid and a. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. Surfactants are molecules, such. Surface Area Of Liquid.
From www.physicsforums.com
Find the surface area of the water in the given prism Surface Area Of Liquid Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. The stronger the intermolecular interactions, the greater the surface tension. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Surface tension is the energy required to increase the surface area of a liquid by. Surface Area Of Liquid.
From slideshare.net
Primary 5 Cycles Water Surface Area Of Liquid This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is involved in. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is defined as the energy required to increase the surface area of a liquid, or. Surface Area Of Liquid.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Surface Area Of Liquid Surface body formulas and calculation. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. The surface area is the amount of that liquid that is. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive. Surface Area Of Liquid.
From www.teachoo.com
Question 4 A hemispherical tank full of water is emptied Surface Area Of Liquid Surface body formulas and calculation. This applies to reactions involving a solid and a gas, or a solid and a. The surface area is the amount of that liquid that is. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. Surface tension. Surface Area Of Liquid.
From infogram.com
Solubility Infographic Infogram Surface Area Of Liquid The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. Surface body formulas and calculation. The surface area is the amount of that liquid that is. This page describes and explains the effect of changing the surface area of a solid on the rate of a reaction it is. Surface Area Of Liquid.
From saylordotorg.github.io
Liquids Surface Area Of Liquid The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. However, the molecules on the. This applies to reactions involving a solid and a gas, or a solid and a. Surface body formulas and calculation. The surface of a liquid is the interface between the liquid and (usually) the. Surface Area Of Liquid.
From www.slideserve.com
PPT Discussion Lecture(4) Chapter(3) Hydrostatic Forces on Surfaces Surface Area Of Liquid However, the molecules on the. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. The stronger the intermolecular interactions, the greater the surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. This page describes and explains the. Surface Area Of Liquid.
From www.researchgate.net
Surface area of water volumefraction between 0.1 and 0.9 Download Surface Area Of Liquid Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. However, the molecules on the. This applies to reactions involving a solid and a gas, or a solid and a. The surface area is the amount of that liquid that is. The molecules. Surface Area Of Liquid.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Surface Area Of Liquid Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The molecules within a liquid are surrounded by other molecules and are attracted equally in. Surface Area Of Liquid.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Surface Area Of Liquid The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Surface tension is the energy required to increase the surface area of a liquid by a given amount. However, the molecules on the. The stronger the intermolecular interactions, the greater the surface tension. This page describes and explains the effect of changing the. Surface Area Of Liquid.
From www.slideserve.com
PPT Pumping Apparatus Driver/Operator — Lesson 6 PowerPoint Surface Area Of Liquid The stronger the intermolecular interactions, the greater the surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. Surface body formulas and calculation. This page describes and explains the effect of changing the. Surface Area Of Liquid.
From www.thoughtco.com
Liquid Definition and Examples (Chemistry) Surface Area Of Liquid The stronger the intermolecular interactions, the greater the surface tension. However, the molecules on the. Surface body formulas and calculation. The surface area is the amount of that liquid that is. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces. This page describes and explains the effect of. Surface Area Of Liquid.
From www.researchgate.net
Surface Area of Water Infiltration and Hardening Download Scientific Surface Area Of Liquid The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The stronger the intermolecular interactions, the greater the surface tension. The surface area is the amount of that liquid that is. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within. Surface Area Of Liquid.
From www.youtube.com
Surface Tension ( PartII )_Behavior of Free Liquid Surface Class XI Surface Area Of Liquid This applies to reactions involving a solid and a gas, or a solid and a. The stronger the intermolecular interactions, the greater the surface tension. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by. Surface Area Of Liquid.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID6584897 Surface Area Of Liquid The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. Surface body formulas and calculation. Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. The surface area is. Surface Area Of Liquid.
From allimportantnotes.com
Class 9 Maths Surface Area and Volumes Notes All Important Notes Surface Area Of Liquid Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. Surface body formulas and calculation. The surface of a liquid is the interface between the liquid and (usually) the air surrounding it. The molecules within a liquid are surrounded by other molecules and. Surface Area Of Liquid.
From pressbooks.bccampus.ca
11.4 Variation of Pressure with Depth in a Fluid College Physics Surface Area Of Liquid Surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid. Surface body formulas and calculation. The stronger the intermolecular interactions, the greater the surface tension. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by. Surface Area Of Liquid.
From poster.4teachers.org
Does The Surface Area of Water Affect The Rate of Evaporation? Surface Area Of Liquid Surface body formulas and calculation. The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The molecules within a liquid are surrounded by other molecules and are attracted. Surface Area Of Liquid.