Differential Heat Of Solution Define . Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Understand how energy changes occur on mixing. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at.
from www.chegg.com
The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Understand how energy changes occur on mixing.
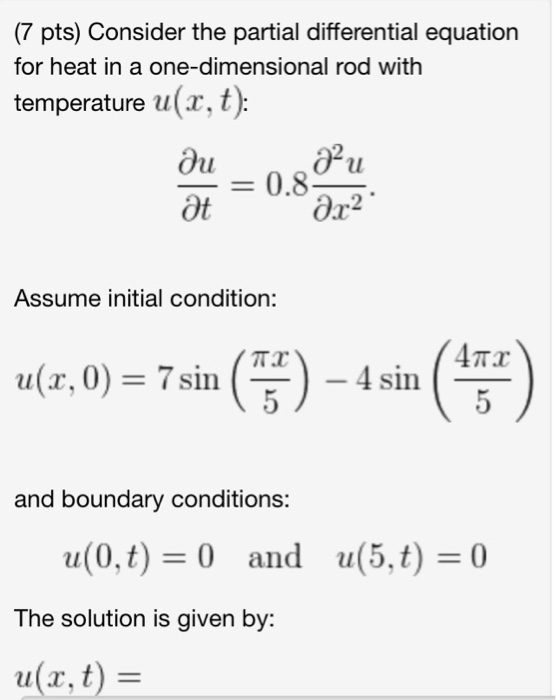
Solved Consider the partial differential equation for heat
Differential Heat Of Solution Define In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Understand how energy changes occur on mixing. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the.
From www.youtube.com
Heat Transfer L12 p1 Finite Difference Heat Equation YouTube Differential Heat Of Solution Define The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or. Differential Heat Of Solution Define.
From www.chegg.com
Solved Differential analysis of heat transfer in the flow Differential Heat Of Solution Define The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. Understand how energy changes occur on mixing. The enthalpy change of solution refers to the amount of heat. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Differential Heat Of Solution Define The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. Understand how. Differential Heat Of Solution Define.
From www.youtube.com
Finite Difference Solution of "Heat conduction in a rod" Explicit Differential Heat Of Solution Define The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Distinguish between integral heat of solution, differential heat of. Differential Heat Of Solution Define.
From www.chegg.com
Solved Consider the partial differential equation for heat Differential Heat Of Solution Define The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute. Differential Heat Of Solution Define.
From exowvkchx.blob.core.windows.net
Definition Differential Heat Of Solution at Rachel Carter blog Differential Heat Of Solution Define Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Understand how energy changes occur on mixing. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Solution Properties PowerPoint Presentation, free download ID Differential Heat Of Solution Define The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The enthalpy change of solution refers to the amount of heat that. Differential Heat Of Solution Define.
From www.youtube.com
CHEM 101 Calculating Enthalpy of Solution 2 YouTube Differential Heat Of Solution Define Understand how energy changes occur on mixing. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution (δh. Differential Heat Of Solution Define.
From exowvkchx.blob.core.windows.net
Definition Differential Heat Of Solution at Rachel Carter blog Differential Heat Of Solution Define The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Understand how energy changes occur on mixing. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\). Differential Heat Of Solution Define.
From www.youtube.com
Deriving the Heat Equation A Parabolic Partial Differential Equation Differential Heat Of Solution Define In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute. Differential Heat Of Solution Define.
From www.youtube.com
What Does It Mean to Solve the Heat Equation PDE? An Introduction with Differential Heat Of Solution Define In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Heat of Solution PowerPoint Presentation, free download ID1550557 Differential Heat Of Solution Define The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. Understand how energy changes occur on mixing. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Heat of Solution PowerPoint Presentation, free download ID1550557 Differential Heat Of Solution Define In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. Understand how energy changes occur on mixing. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving. Differential Heat Of Solution Define.
From exowvkchx.blob.core.windows.net
Definition Differential Heat Of Solution at Rachel Carter blog Differential Heat Of Solution Define The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Understand how energy changes occur on mixing. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Distinguish between. Differential Heat Of Solution Define.
From owlcation.com
Differential Equations Owlcation Differential Heat Of Solution Define Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. In thermochemistry,. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Heat of Solution PowerPoint Presentation, free download ID1550557 Differential Heat Of Solution Define Understand how energy changes occur on mixing. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released. Differential Heat Of Solution Define.
From www.sliderbase.com
Formation of Solutions Presentation Chemistry Differential Heat Of Solution Define The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Distinguish between integral heat of solution, differential heat of. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Enthalpy Changes in Solution PowerPoint Presentation, free Differential Heat Of Solution Define Understand how energy changes occur on mixing. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Distinguish between integral heat of solution, differential heat of. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID1709175 Differential Heat Of Solution Define The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. Understand how energy changes occur on mixing. The enthalpy change of solution. Differential Heat Of Solution Define.
From www.researchgate.net
Differential heatheat weight map of LCP1. Download Scientific Diagram Differential Heat Of Solution Define The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Understand how energy changes occur on mixing. Distinguish between integral heat. Differential Heat Of Solution Define.
From www.slideserve.com
PPT 4. Advanced chemical thermodynamics PowerPoint Presentation, free Differential Heat Of Solution Define The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. Understand how. Differential Heat Of Solution Define.
From www.chemistrylearner.com
Heat (Enthalpy) of Solution Definition, Formula, & Problems Differential Heat Of Solution Define The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution (δh soln) is the heat released or. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Heat Equations of Change I PowerPoint Presentation, free download Differential Heat Of Solution Define Understand how energy changes occur on mixing. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed. Differential Heat Of Solution Define.
From exowvkchx.blob.core.windows.net
Definition Differential Heat Of Solution at Rachel Carter blog Differential Heat Of Solution Define The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The molar. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Heat Equations of Change I PowerPoint Presentation, free download Differential Heat Of Solution Define Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The enthalpy change. Differential Heat Of Solution Define.
From www.doubtnut.com
[Odia] Define molar differential enthalpy of solution. Differential Heat Of Solution Define The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution (δh soln) is the heat released or. Differential Heat Of Solution Define.
From www.youtube.com
Heating Value Solution Differential Equations in Action YouTube Differential Heat Of Solution Define Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Understand how energy changes occur on mixing. The enthalpy change of solution refers to the amount of heat. Differential Heat Of Solution Define.
From chempedia.info
Integral and Differential Heats of Solution Big Chemical Encyclopedia Differential Heat Of Solution Define The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy of solution (δh soln) is the heat released or. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation Differential Heat Of Solution Define The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Understand how energy changes occur on mixing. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The enthalpy change of solution refers to the amount of. Differential Heat Of Solution Define.
From www.researchgate.net
3 Actuation principles based on a) differential heating of Differential Heat Of Solution Define The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Understand how energy changes occur on mixing. Distinguish between integral heat. Differential Heat Of Solution Define.
From www.slideserve.com
PPT 4. Advanced chemical thermodynamics PowerPoint Presentation, free Differential Heat Of Solution Define The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of the. Differential Heat Of Solution Define.
From www.youtube.com
HEAT EQUATION PARTIAL DIFFERENTIAL EQUATION PROBLEM 3 YouTube Differential Heat Of Solution Define The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute. Differential Heat Of Solution Define.
From www.slideserve.com
PPT 4. Advanced chemical thermodynamics PowerPoint Presentation, free Differential Heat Of Solution Define Distinguish between integral heat of solution, differential heat of solution, heat of solution at. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Understand how energy changes occur on mixing. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated. Differential Heat Of Solution Define.
From www.slideserve.com
PPT Enthalpy Changes in Solution PowerPoint Presentation, free Differential Heat Of Solution Define The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. The molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed. Differential Heat Of Solution Define.
From www.sharetechnote.com
Engineering Math ShareTechnote Differential Heat Of Solution Define Distinguish between integral heat of solution, differential heat of solution, heat of solution at. In thermochemistry, the enthalpy of solution (heat of solution or enthalpy of solvation) is the enthalpy change associated with the. The enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain. The enthalpy change. Differential Heat Of Solution Define.