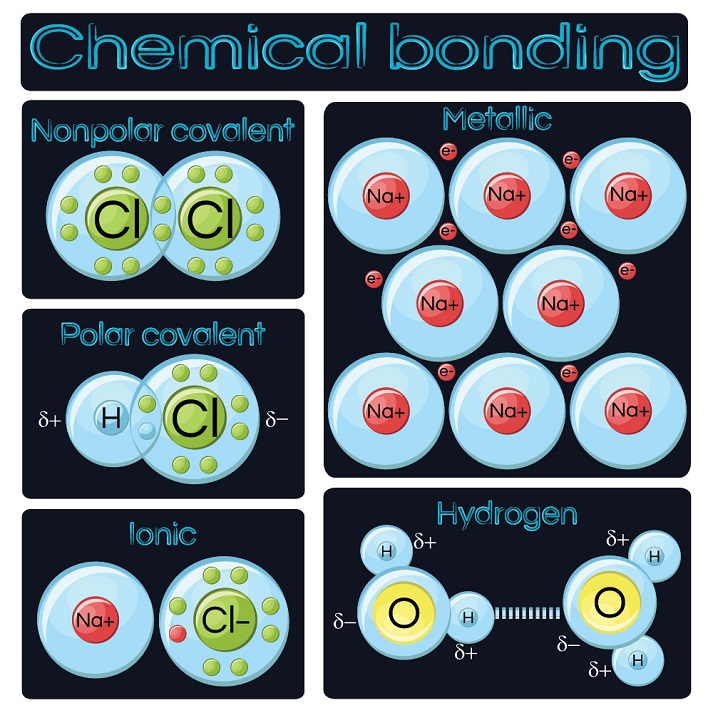
Bind Vs Bond In Chemistry . Two models have been developed to describe covalent bonding: Bond order and length are inversely. Bond order and bond length indicate the type and strength of covalent bonds between atoms. Bond energy is defined as the energy required to break a. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. Each model has its strengths and weaknesses, and chemists. Valence bond theory and molecular orbital theory. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Mechanically bonded molecules typically fall into two broad categories. Bond strengths increase as bond order increases, while bond distances decrease. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,.
from mungfali.com
Bond strengths increase as bond order increases, while bond distances decrease. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Bond order and bond length indicate the type and strength of covalent bonds between atoms. Each model has its strengths and weaknesses, and chemists. Two models have been developed to describe covalent bonding: If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Mechanically bonded molecules typically fall into two broad categories. Valence bond theory and molecular orbital theory. Bond energy is defined as the energy required to break a.
Chemical Bonding Chart
Bind Vs Bond In Chemistry Bond strengths increase as bond order increases, while bond distances decrease. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Two models have been developed to describe covalent bonding: Bond order and bond length indicate the type and strength of covalent bonds between atoms. Valence bond theory and molecular orbital theory. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Bond energy is defined as the energy required to break a. Bond order and length are inversely. Each model has its strengths and weaknesses, and chemists. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. Mechanically bonded molecules typically fall into two broad categories. Bond strengths increase as bond order increases, while bond distances decrease.
From mungfali.com
Chemical Bonding Chart Bind Vs Bond In Chemistry Each model has its strengths and weaknesses, and chemists. Bond strengths increase as bond order increases, while bond distances decrease. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Two models have been developed to describe covalent bonding: Valence bond theory and molecular orbital theory. Mechanically bonded molecules. Bind Vs Bond In Chemistry.
From www.britannica.com
crystal Types of bonds Britannica Bind Vs Bond In Chemistry Each model has its strengths and weaknesses, and chemists. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Two models have been developed to describe covalent bonding: Bond strengths increase as bond order increases, while bond. Bind Vs Bond In Chemistry.
From slideplayer.com
Nomenclature & Chemical Bonding ppt download Bind Vs Bond In Chemistry Bond order and length are inversely. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,.. Bind Vs Bond In Chemistry.
From generalideasinchemistryforbegginners.blogspot.com
GENERAL IDEAS IN CHEMISTRY FOR BEGINNERS COVALENT BONDS And DATIVE Bind Vs Bond In Chemistry Bond strengths increase as bond order increases, while bond distances decrease. Each model has its strengths and weaknesses, and chemists. Valence bond theory and molecular orbital theory. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. Chemical bonds form when electrons can be simultaneously close to two or more. Bind Vs Bond In Chemistry.
From science4fun.info
Chemical Bonding (Types, Formation, and Facts) Science4Fun Bind Vs Bond In Chemistry Valence bond theory and molecular orbital theory. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Bond energy is defined as the energy required to break a. If a linear molecule is threaded through a macrocycle. Bind Vs Bond In Chemistry.
From pathwaystochemistry.com
Table of Bond Energies Pathways to Chemistry Bind Vs Bond In Chemistry If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. Each model has its strengths and weaknesses,. Bind Vs Bond In Chemistry.
From www.youtube.com
Types of Bonding Primary bonds & Secondary bonds YouTube Bind Vs Bond In Chemistry If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Each model has its strengths and weaknesses, and chemists. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked. Bind Vs Bond In Chemistry.
From www.chemistrylearner.com
Hydrogen Bond Definition, Types, and Examples Bind Vs Bond In Chemistry Bond strengths increase as bond order increases, while bond distances decrease. Two models have been developed to describe covalent bonding: Valence bond theory and molecular orbital theory. Mechanically bonded molecules typically fall into two broad categories. Bond order and length are inversely. Each model has its strengths and weaknesses, and chemists. Bond order and bond length indicate the type and. Bind Vs Bond In Chemistry.
From www.tec-science.com
Covalent bonding tecscience Bind Vs Bond In Chemistry If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Bond energy is defined as the energy required to break a. Mechanically bonded molecules typically fall into two broad categories. Bond strengths increase as bond order increases,. Bind Vs Bond In Chemistry.
From hubpages.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Bind Vs Bond In Chemistry Bond strengths increase as bond order increases, while bond distances decrease. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Bond order and length are inversely. Bond order and bond length indicate the type and strength. Bind Vs Bond In Chemistry.
From thecontentauthority.com
Bind vs Bond Meaning And Differences Bind Vs Bond In Chemistry If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. Bond strengths increase as bond order increases,. Bind Vs Bond In Chemistry.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Bind Vs Bond In Chemistry Two models have been developed to describe covalent bonding: Each model has its strengths and weaknesses, and chemists. Bond energy is defined as the energy required to break a. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. Valence bond theory and molecular orbital theory. If the total energy. Bind Vs Bond In Chemistry.
From ar.inspiredpencil.com
Polar Covalent Bond Vs Nonpolar Covalent Bond Bind Vs Bond In Chemistry If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. Mechanically bonded molecules typically fall into two broad categories. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Each model has its strengths and weaknesses, and chemists. Two. Bind Vs Bond In Chemistry.
From www.biologyonline.com
Covalent bond Definition and Examples Biology Online Dictionary Bind Vs Bond In Chemistry If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Bond strengths increase as bond order. Bind Vs Bond In Chemistry.
From www.britannica.com
Covalent bond Definition, Properties, Examples, & Facts Britannica Bind Vs Bond In Chemistry Valence bond theory and molecular orbital theory. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Each model has its strengths and weaknesses, and chemists. Mechanically bonded molecules typically fall into two broad categories. Two models have been developed to describe covalent bonding: Bond order and bond length. Bind Vs Bond In Chemistry.
From www.ownguru.com
concept of Chemical bond Bind Vs Bond In Chemistry Mechanically bonded molecules typically fall into two broad categories. Bond order and bond length indicate the type and strength of covalent bonds between atoms. Each model has its strengths and weaknesses, and chemists. Valence bond theory and molecular orbital theory. If the total energy of a group of atoms is lower than the sum of the energies of the component. Bind Vs Bond In Chemistry.
From www.youtube.com
Chemistry Covalent bond, Bond pair and Lone pair Chemical Bonding Bind Vs Bond In Chemistry Bond strengths increase as bond order increases, while bond distances decrease. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Two models have been developed to describe covalent bonding: Bond energy is defined as the energy required to break a. Mechanically bonded molecules typically fall into two broad. Bind Vs Bond In Chemistry.
From www.researchgate.net
CO (A) and CO 2 (B) bonding to transition metals. (A) CO binds to a Bind Vs Bond In Chemistry If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Two models have been developed to describe covalent bonding: Bond strengths increase as bond order increases, while bond distances. Bind Vs Bond In Chemistry.
From www.scribd.com
Chapter Three Crystal Binding Why Do Atoms Form Crystals or Solids Bind Vs Bond In Chemistry If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. If a linear molecule is threaded. Bind Vs Bond In Chemistry.
From chem.libretexts.org
4.11 Multiple Bonds in MO Theory Chemistry LibreTexts Bind Vs Bond In Chemistry If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the. Each model has its strengths and weaknesses, and chemists. Bond order and length are inversely. Valence bond theory and molecular orbital theory. Two models have been developed to describe covalent bonding: Bond energy is defined as the energy required to. Bind Vs Bond In Chemistry.
From theoryanalysis.netlify.app
Chemical bond chemical bonds Bind Vs Bond In Chemistry Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Each model has its strengths and weaknesses, and chemists. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is. Bind Vs Bond In Chemistry.
From socratic.com
Bonding Chemistry Socratic Bind Vs Bond In Chemistry Bond order and bond length indicate the type and strength of covalent bonds between atoms. Bond order and length are inversely. Mechanically bonded molecules typically fall into two broad categories. Two models have been developed to describe covalent bonding: Bond energy is defined as the energy required to break a. Bond strengths increase as bond order increases, while bond distances. Bind Vs Bond In Chemistry.
From www.chemistrysteps.com
Bond Length and Bond Strength Chemistry Steps Bind Vs Bond In Chemistry Bond strengths increase as bond order increases, while bond distances decrease. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Each model has its strengths and weaknesses, and chemists. Mechanically bonded molecules typically fall into two broad categories. Bond energy is defined as the energy required to break. Bind Vs Bond In Chemistry.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Bind Vs Bond In Chemistry Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Bond order and bond length indicate the type and strength of covalent bonds between atoms. Bond strengths increase as bond order increases, while bond distances decrease. If the total energy of a group of atoms is lower than the. Bind Vs Bond In Chemistry.
From www.thoughtco.com
Bonds Definition and Examples in Chemistry Bind Vs Bond In Chemistry Two models have been developed to describe covalent bonding: Valence bond theory and molecular orbital theory. Each model has its strengths and weaknesses, and chemists. Bond order and bond length indicate the type and strength of covalent bonds between atoms. Bond strengths increase as bond order increases, while bond distances decrease. Chemical bonds form when electrons can be simultaneously close. Bind Vs Bond In Chemistry.
From www.britannica.com
Metallic bond Properties, Examples, & Explanation Britannica Bind Vs Bond In Chemistry Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Bond energy is defined as the energy required to break a. Each model has its strengths and weaknesses, and chemists. Bond order and bond length indicate the type and strength of covalent bonds between atoms. Valence bond theory and. Bind Vs Bond In Chemistry.
From overallscience.com
Forms of Binding in Crystals Overall Science Bind Vs Bond In Chemistry Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Mechanically bonded molecules typically fall into. Bind Vs Bond In Chemistry.
From www.chemistrysteps.com
Bond Length and Bond Strength Chemistry Steps Bind Vs Bond In Chemistry If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Bond energy is defined as the energy required to break a. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair. Bind Vs Bond In Chemistry.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together Bind Vs Bond In Chemistry Bond order and length are inversely. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Valence bond theory and molecular orbital theory. Each model has its strengths and weaknesses, and chemists. Mechanically bonded molecules typically fall. Bind Vs Bond In Chemistry.
From www.youtube.com
Types Of Chemical Bonds What Are Chemical Bonds Covalent Bonds And Bind Vs Bond In Chemistry Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Mechanically bonded molecules typically fall into two broad categories. Bond strengths increase as bond order increases, while bond distances decrease. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings,. Bind Vs Bond In Chemistry.
From med.libretexts.org
2.2A Covalent Bonds and Other Bonds and Interactions Medicine LibreTexts Bind Vs Bond In Chemistry Each model has its strengths and weaknesses, and chemists. Mechanically bonded molecules typically fall into two broad categories. Bond strengths increase as bond order increases, while bond distances decrease. Bond order and length are inversely. If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together. Bind Vs Bond In Chemistry.
From sciencenotes.org
Types of Chemical Bonds Bind Vs Bond In Chemistry Bond order and bond length indicate the type and strength of covalent bonds between atoms. Two models have been developed to describe covalent bonding: If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Bond energy is. Bind Vs Bond In Chemistry.
From www.youtube.com
Chemistry Lesson 31 Different Types of Chemical Bonds YouTube Bind Vs Bond In Chemistry If the total energy of a group of atoms is lower than the sum of the energies of the component atoms, they then bond together and the energy lowering is the bonding energy. Bond order and bond length indicate the type and strength of covalent bonds between atoms. Bond energy is defined as the energy required to break a. If. Bind Vs Bond In Chemistry.
From www.youtube.com
Basic Chemistry for Biology Part 4 Covalent Bonding and Structural Bind Vs Bond In Chemistry Valence bond theory and molecular orbital theory. Each model has its strengths and weaknesses, and chemists. Bond energy is defined as the energy required to break a. Bond strengths increase as bond order increases, while bond distances decrease. Bond order and bond length indicate the type and strength of covalent bonds between atoms. Two models have been developed to describe. Bind Vs Bond In Chemistry.
From surfguppy.com
Electronegativity Bond Scale Surfguppy Chemistry made easy for Bind Vs Bond In Chemistry Bond order and length are inversely. Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. Valence bond theory and molecular orbital theory. Two models have been developed to describe covalent bonding: Mechanically bonded molecules typically fall into two broad categories. If a linear molecule is threaded through a. Bind Vs Bond In Chemistry.