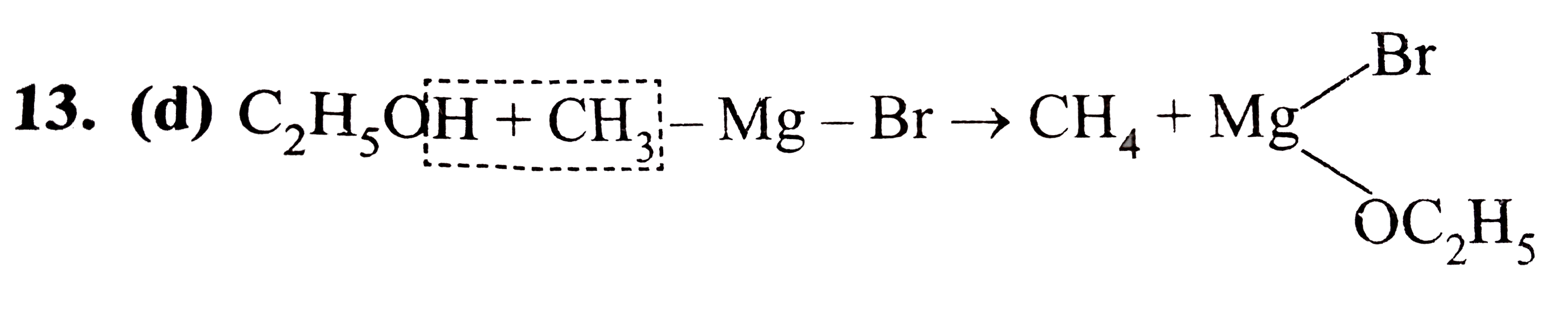Methyl Magnesium Bromide With Ethyl Alcohol . Primary and secondary alcohols are much m. Methyl group in the grignard reagent has a lone pair with a. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). Here we cover eight different examples of their reactions. the order of reactivity of alcohols is 3° > 2° > 1° methyl. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. methyl magnesium bromide reacts with ethanol to give methane.
from www.doubtnut.com
Methyl group in the grignard reagent has a lone pair with a. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. methyl magnesium bromide reacts with ethanol to give methane. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. Primary and secondary alcohols are much m. the order of reactivity of alcohols is 3° > 2° > 1° methyl.
A reaction between methyl magnesium bromide and ethyl alcohol gives
Methyl Magnesium Bromide With Ethyl Alcohol the order of reactivity of alcohols is 3° > 2° > 1° methyl. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. Primary and secondary alcohols are much m. Methyl group in the grignard reagent has a lone pair with a. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). Here we cover eight different examples of their reactions. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. methyl magnesium bromide reacts with ethanol to give methane. the order of reactivity of alcohols is 3° > 2° > 1° methyl. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol.
From www.youtube.com
Methyl magnesium bromide is treated with ethanol, what is the formu... YouTube Methyl Magnesium Bromide With Ethyl Alcohol Methyl group in the grignard reagent has a lone pair with a. methyl magnesium bromide reacts with ethanol to give methane. Primary and secondary alcohols are much m. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.drsebiscellfood.com
Bromide Plus Powder Dr. Sebi's Cell Food Methyl Magnesium Bromide With Ethyl Alcohol The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Methyl group in the grignard. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
4) CH, 88. Reaction between dry ice (solid CO,) and methyl magnesium bromide gives an addition Methyl Magnesium Bromide With Ethyl Alcohol Methyl group in the grignard reagent has a lone pair with a. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. the order of reactivity of alcohols is 3° > 2° > 1° methyl. — the grignard reagents are formed from the reaction of an alkyl halide. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
Ethyl acetate is obtained when methyl magnesium bromide reacts with Methyl Magnesium Bromide With Ethyl Alcohol Here we cover eight different examples of their reactions. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. the reaction of methyl magnesium bromide with acetone followed by. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
An ester A (C4H8O2) , on treatment with excess of methyl magnesium bromide followed by Methyl Magnesium Bromide With Ethyl Alcohol tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. Primary and secondary alcohols are. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Methyl Magnesium Bromide With Ethyl Alcohol the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. Primary and secondary alcohols are much m. Here we cover eight different examples of their reactions. the order of reactivity of alcohols is 3°. Methyl Magnesium Bromide With Ethyl Alcohol.
From byjus.com
38. What is the Reaction equation of methyl magnesium bromide reacting with oxygen. Methyl Magnesium Bromide With Ethyl Alcohol the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. methyl magnesium bromide reacts with ethanol to give methane. — grignard reagents are formed by the reaction of magnesium metal with alkyl or. Methyl Magnesium Bromide With Ethyl Alcohol.
From exogzetoh.blob.core.windows.net
Methyl Magnesium Bromide Wiki at Dustin Coats blog Methyl Magnesium Bromide With Ethyl Alcohol — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. Here we cover eight different examples of their reactions. — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion Methyl Magnesium Bromide With Ethyl Alcohol — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. Here we cover eight different examples of their reactions. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. Primary and secondary alcohols are much m. The order of reactivity. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.doubtnut.com
A reaction between methyl magnesium bromide and ethyl alcohol gives Methyl Magnesium Bromide With Ethyl Alcohol — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. methyl magnesium bromide reacts with ethanol to give methane. the order of reactivity of alcohols is 3° > 2° >. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
How are the following conversions carried out?(i) Propene Propan 2 ol(ii) Benzyl chloride Methyl Magnesium Bromide With Ethyl Alcohol — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). methyl magnesium bromide reacts with ethanol to give methane. the order of reactivity of alcohols is 3° > 2° > 1° methyl.. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
Which of the following are the intermediate in the reaction of excess methyl magnesium bromide Methyl Magnesium Bromide With Ethyl Alcohol — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. The order of reactivity of the hydrogen halides. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Illustration of stuff, alcohol Methyl Magnesium Bromide With Ethyl Alcohol — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. Primary and secondary alcohols are much m. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. Methyl group in the grignard reagent has a lone pair with a. — grignard reagents are formed. Methyl Magnesium Bromide With Ethyl Alcohol.
From sielc.com
Vinyl bromide SIELC Technologies Methyl Magnesium Bromide With Ethyl Alcohol Primary and secondary alcohols are much m. the order of reactivity of alcohols is 3° > 2° > 1° methyl. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. methyl magnesium bromide reacts with ethanol to give methane. — the grignard reagents are formed from the. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.quora.com
What is the reaction of methyl magnesium bromide on ethyl cyanide? Quora Methyl Magnesium Bromide With Ethyl Alcohol The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). Here we cover eight different examples of their reactions. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. Methyl group in the grignard reagent has a lone pair with a. . Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
The reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary Methyl Magnesium Bromide With Ethyl Alcohol the order of reactivity of alcohols is 3° > 2° > 1° methyl. Methyl group in the grignard reagent has a lone pair with a. — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Primary and secondary alcohols are much m. tertiary alcohols react with either hcl or hbr at. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.fishersci.com
Alfa Aesar Bromoacetyl bromide, 98 Fisher Scientific Methyl Magnesium Bromide With Ethyl Alcohol Methyl group in the grignard reagent has a lone pair with a. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. — however, there’s a twist with the. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.doubtnut.com
Carry out the synthesis of the following alcohols from ethyl magnesium Methyl Magnesium Bromide With Ethyl Alcohol — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. methyl magnesium bromide reacts with ethanol to give methane. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. the order of reactivity of alcohols is 3° > 2° > 1°. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Methyl Magnesium Bromide With Ethyl Alcohol Primary and secondary alcohols are much m. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Methyl group in the grignard reagent has a lone pair with a. the order of. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
An ester A (C4H8O2) , on treatment with excess of methyl magnesium bromide followed by Methyl Magnesium Bromide With Ethyl Alcohol Primary and secondary alcohols are much m. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
What happens when formaldehyde is treated with methyl magnesium bromide and the product is then Methyl Magnesium Bromide With Ethyl Alcohol — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Methyl group in the grignard reagent has a lone pair with a. methyl magnesium bromide reacts with ethanol to give methane. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. the order. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Methyl Magnesium Bromide With Ethyl Alcohol methyl magnesium bromide reacts with ethanol to give methane. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. the reaction of methyl magnesium bromide with acetone followed by hydrolysis. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
) TP 15, 7. A reaction between methyl magnesium bromide and ethyl alcohol gives (1) Methane (2 Methyl Magnesium Bromide With Ethyl Alcohol The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Primary and secondary alcohols are much m. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones.. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Methyl Magnesium Bromide With Ethyl Alcohol — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. Methyl group in the grignard reagent has a lone pair with a. Primary and secondary alcohols are much m. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. The order of. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
The reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary Methyl Magnesium Bromide With Ethyl Alcohol Here we cover eight different examples of their reactions. Primary and secondary alcohols are much m. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. tertiary alcohols react with either. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
) TP 15, 7. A reaction between methyl magnesium bromide and ethyl alcohol gives (1) Methane (2 Methyl Magnesium Bromide With Ethyl Alcohol — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. the order of reactivity of alcohols. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
The reaction of methyl magnesium bromide reacts with acetone followed by hydrolysis gives Methyl Magnesium Bromide With Ethyl Alcohol tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). the order of reactivity of alcohols is 3° > 2° > 1° methyl. Primary and secondary alcohols are much m. . Methyl Magnesium Bromide With Ethyl Alcohol.
From www.doubtnut.com
What happens when methanal is treated with methyl megnesium bromide an Methyl Magnesium Bromide With Ethyl Alcohol tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. Methyl group in the grignard reagent has a lone pair with a. methyl magnesium bromide reacts with ethanol to give methane. — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Here. Methyl Magnesium Bromide With Ethyl Alcohol.
From molekula.com
Purchase Methylmagnesium bromide 1M in THF [75161] online • Catalog • Molekula Group Methyl Magnesium Bromide With Ethyl Alcohol Primary and secondary alcohols are much m. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). methyl magnesium bromide reacts with ethanol to give methane. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. Here we cover eight different examples of their reactions. . Methyl Magnesium Bromide With Ethyl Alcohol.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Methyl Magnesium Bromide With Ethyl Alcohol the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. methyl magnesium bromide reacts with ethanol. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.youtube.com
methyl magnesium bromide(grignard reagent) ethyl alcohol ke saath kriya karke deta hai ? YouTube Methyl Magnesium Bromide With Ethyl Alcohol the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. the order of reactivity of alcohols is 3° > 2° > 1° methyl. tertiary alcohols react with either hcl or hbr at 0 °c by an sn1 mechanism through a carbocation intermediate. The order of reactivity of the hydrogen halides is hi >. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.toppr.com
The organic reaction product from the reaction of methyl magnesium bromide and ethyl alcohol is Methyl Magnesium Bromide With Ethyl Alcohol The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). Here we cover eight different examples of their reactions. the order of reactivity of alcohols is 3° > 2° > 1° methyl. methyl magnesium bromide reacts with ethanol to give methane. Methyl group in the grignard reagent has a lone. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.doubtnut.com
A reaction between methyl magnesium bromide and ethyl alcohol gives Methyl Magnesium Bromide With Ethyl Alcohol methyl magnesium bromide reacts with ethanol to give methane. — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. the reaction of methyl magnesium bromide with acetone followed by hydrolysis gives secondary alcohol. Primary and secondary alcohols are much m. — the grignard reagents are formed from the reaction of. Methyl Magnesium Bromide With Ethyl Alcohol.
From exogzetoh.blob.core.windows.net
Methyl Magnesium Bromide Wiki at Dustin Coats blog Methyl Magnesium Bromide With Ethyl Alcohol methyl magnesium bromide reacts with ethanol to give methane. Primary and secondary alcohols are much m. — grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. the order of reactivity of alcohols is 3° > 2° > 1° methyl. — however, there’s a twist with the reaction of esters that. Methyl Magnesium Bromide With Ethyl Alcohol.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Methyl Magnesium Bromide With Ethyl Alcohol — the grignard reagents are formed from the reaction of an alkyl halide with magnesium metal in anhydrous ether. — however, there’s a twist with the reaction of esters that isn’t present with aldehydes and ketones. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is generally unreactive). tertiary alcohols react. Methyl Magnesium Bromide With Ethyl Alcohol.