Zinc Chloride Dissolved In Water . to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. The polar water molecules are. — yes, zinc chloride (zncl2) is soluble in water. The chemical equation for this reaction is given by: With excess water, zinc oxychlorides are formed. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. — in this video we will describe the equation zncl2 + h2o and write what. zinc chloride is very soluble in water & will usually form clear solution. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl As potassium chloride (kcl) dissolves in water, the ions are hydrated. 1 2 it readily dissolves in water to form a clear, colorless solution. 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if.
from www.bartleby.com
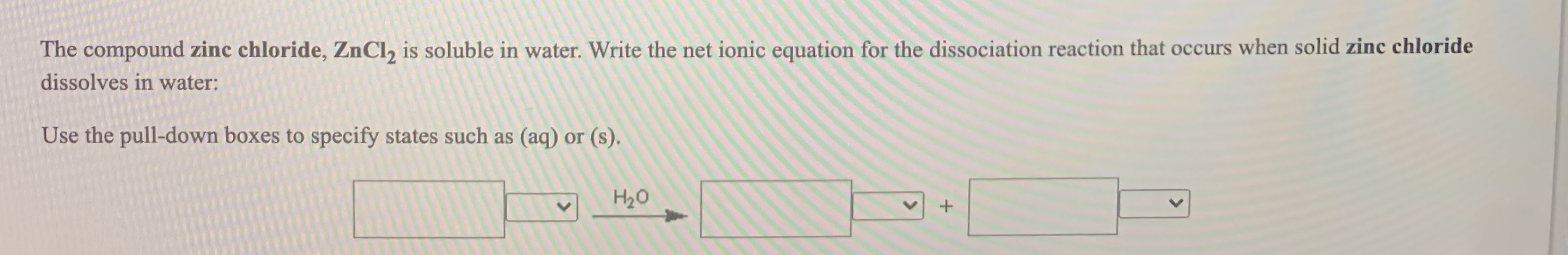
to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. As potassium chloride (kcl) dissolves in water, the ions are hydrated. The polar water molecules are. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl — in this video we will describe the equation zncl2 + h2o and write what. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. — yes, zinc chloride (zncl2) is soluble in water. With excess water, zinc oxychlorides are formed. The chemical equation for this reaction is given by: zinc chloride is very soluble in water & will usually form clear solution.
Answered The compound zinc chloride, ZnCl, is… bartleby
Zinc Chloride Dissolved In Water Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl The polar water molecules are. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. — yes, zinc chloride (zncl2) is soluble in water. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. zinc chloride is very soluble in water & will usually form clear solution. 1 2 it readily dissolves in water to form a clear, colorless solution. With excess water, zinc oxychlorides are formed. The chemical equation for this reaction is given by: As potassium chloride (kcl) dissolves in water, the ions are hydrated. — in this video we will describe the equation zncl2 + h2o and write what.
From camachem.com
What is Zinc Chloride and How to Buy Zinc Chloride? Zinc Chloride Dissolved In Water Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl 1 2 it readily dissolves in water to form a clear, colorless solution. The chemical equation for this reaction is given by: — in this video we will describe the equation zncl2 + h2o and write what. The polar water molecules are. As potassium chloride (kcl) dissolves. Zinc Chloride Dissolved In Water.
From www.youtube.com
Is ZnCl2 Soluble or Insoluble in Water? YouTube Zinc Chloride Dissolved In Water The chemical equation for this reaction is given by: — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl The polar water molecules are. — yes, zinc chloride (zncl2) is soluble in water. 1 2 it readily dissolves. Zinc Chloride Dissolved In Water.
From www.flinnsci.com
Flinn Chemicals, Zinc Chloride Zinc Chloride Dissolved In Water The polar water molecules are. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl zinc chloride is very soluble in water & will usually form clear solution. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. to prepare 1000 ml of a 0.1 mol/l solution. Zinc Chloride Dissolved In Water.
From exorueyvz.blob.core.windows.net
Zinc Chloride Density Water at Rolando Burgan blog Zinc Chloride Dissolved In Water 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. As potassium chloride (kcl) dissolves in water, the ions are hydrated. With excess water, zinc oxychlorides are formed. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl to prepare 1000 ml of a 0.1 mol/l. Zinc Chloride Dissolved In Water.
From mungfali.com
Zinc Chloride Solution Zinc Chloride Dissolved In Water — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl The polar water molecules are. — yes, zinc chloride (zncl2) is soluble in water. With excess water, zinc oxychlorides are formed. 133 rows — — in general. Zinc Chloride Dissolved In Water.
From jasonstark.com
Empirical Formula Lab Zinc Chloride Stark Science Zinc Chloride Dissolved In Water Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. The polar water molecules are. 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. to prepare. Zinc Chloride Dissolved In Water.
From www.numerade.com
SOLVED Zinc metal dissolves in hydrochloric acid to yield hydrogen gas and an aqueous solution Zinc Chloride Dissolved In Water The polar water molecules are. As potassium chloride (kcl) dissolves in water, the ions are hydrated. — in this video we will describe the equation zncl2 + h2o and write what. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. The chemical equation for this reaction is given by:. Zinc Chloride Dissolved In Water.
From tribioscience.com
0.2M Zinc Chloride Solution Tribioscience Zinc Chloride Dissolved In Water The polar water molecules are. zinc chloride is very soluble in water & will usually form clear solution. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. The chemical equation for this reaction is given by: Zncl 2.2h 2 o →. Zinc Chloride Dissolved In Water.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Zinc Chloride Dissolved In Water — in this video we will describe the equation zncl2 + h2o and write what. As potassium chloride (kcl) dissolves in water, the ions are hydrated. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. With excess water, zinc oxychlorides are formed. The polar water molecules are. 1 2. Zinc Chloride Dissolved In Water.
From openoregon.pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Zinc Chloride Dissolved In Water — yes, zinc chloride (zncl2) is soluble in water. As potassium chloride (kcl) dissolves in water, the ions are hydrated. With excess water, zinc oxychlorides are formed. 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. Zncl 2.2h 2 o → zncl(oh) + h 2 o +. Zinc Chloride Dissolved In Water.
From www.bartleby.com
Answered The compound zinc chloride, ZnCl, is… bartleby Zinc Chloride Dissolved In Water — yes, zinc chloride (zncl2) is soluble in water. 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. 1 2 it readily dissolves in water to form a clear, colorless solution. As potassium chloride (kcl) dissolves in water, the ions are hydrated. The polar water molecules are.. Zinc Chloride Dissolved In Water.
From www.chegg.com
Solved Suppose 21.1 g of zinc chloride is dissolved in Zinc Chloride Dissolved In Water 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. The polar water molecules are. — in this video we will describe the equation zncl2 + h2o and write what. As potassium chloride (kcl) dissolves in water, the ions are hydrated. zinc chloride is very soluble in. Zinc Chloride Dissolved In Water.
From www.chegg.com
Solved Suppose 0.423 g of zinc chloride is dissolved in 200. Zinc Chloride Dissolved In Water — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. zinc chloride is very soluble in water & will usually form clear solution. The chemical equation for this reaction is given by: 1 2 it readily dissolves in water to form a clear, colorless solution. Zncl 2.2h 2 o →. Zinc Chloride Dissolved In Water.
From www.chegg.com
Solved Suppose 0.238 g of zinc chloride is dissolved in Zinc Chloride Dissolved In Water Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. The chemical equation for this reaction is given by: 1 2 it readily dissolves in water to form a clear, colorless solution. to prepare 1000 ml. Zinc Chloride Dissolved In Water.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Photographs The Art of Zinc Chloride Dissolved In Water — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl The polar water molecules are. As potassium chloride (kcl) dissolves in water, the ions are hydrated. — yes, zinc chloride (zncl2) is soluble in water. The chemical equation. Zinc Chloride Dissolved In Water.
From www.chegg.com
Solved Suppose 0.555 g of zinc chloride is dissolved in Zinc Chloride Dissolved In Water — in this video we will describe the equation zncl2 + h2o and write what. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. As potassium chloride (kcl) dissolves in water, the ions are hydrated.. Zinc Chloride Dissolved In Water.
From www.shutterstock.com
122 Zinc Chloride Images, Stock Photos & Vectors Shutterstock Zinc Chloride Dissolved In Water to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. — in this video we will describe the equation zncl2 + h2o and write what. As potassium chloride (kcl) dissolves in water, the ions are hydrated. zinc chloride is very soluble. Zinc Chloride Dissolved In Water.
From sciencelab.co.ke
Zinc Chloride Sciencelab limited Zinc Chloride Dissolved In Water 1 2 it readily dissolves in water to form a clear, colorless solution. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl The polar water molecules are. With excess water, zinc oxychlorides are formed. The chemical equation for this reaction is given by: zinc chloride is very soluble in water & will usually form clear solution.. Zinc Chloride Dissolved In Water.
From www.shutterstock.com
Zinc Chloride Formula Zncl2 Cl2zn White ilustración de stock 769733368 Shutterstock Zinc Chloride Dissolved In Water As potassium chloride (kcl) dissolves in water, the ions are hydrated. to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. With excess water,. Zinc Chloride Dissolved In Water.
From www.researchgate.net
What is the proper way to prepare a zinc chloride containing buffer for enzyme analysis Zinc Chloride Dissolved In Water Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. — in this video we will describe the equation zncl2 + h2o and write what. zinc chloride is very soluble in water & will usually form clear. Zinc Chloride Dissolved In Water.
From www.chegg.com
Solved Suppose 40.7 g of zinc chloride is dissolved in Zinc Chloride Dissolved In Water Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl The chemical equation for this reaction is given by: — in this video we will describe the equation zncl2 + h2o and write what. The polar water molecules are. — yes, zinc chloride (zncl2) is soluble in water. zinc chloride is very soluble in water. Zinc Chloride Dissolved In Water.
From allchemical.com.au
Zinc Chloride All Chemical Manufacturing & Consultancy Zinc Chloride Dissolved In Water The chemical equation for this reaction is given by: to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. The polar water. Zinc Chloride Dissolved In Water.
From www.numerade.com
SOLVED In the laboratory YOu dissolve 22.6 g of zinc chloride in a volumetric flask and add Zinc Chloride Dissolved In Water 1 2 it readily dissolves in water to form a clear, colorless solution. With excess water, zinc oxychlorides are formed. As potassium chloride (kcl) dissolves in water, the ions are hydrated. The chemical equation for this reaction is given by: — yes, zinc chloride (zncl2) is soluble in water. to prepare 1000 ml of a 0.1 mol/l solution. Zinc Chloride Dissolved In Water.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Photographs The Art of Zinc Chloride Dissolved In Water The polar water molecules are. 1 2 it readily dissolves in water to form a clear, colorless solution. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. — in this video we will describe the equation zncl2 + h2o and write what. 133 rows — — in general. Zinc Chloride Dissolved In Water.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Photographs The Art of Zinc Chloride Dissolved In Water With excess water, zinc oxychlorides are formed. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. The polar water molecules are. — yes, zinc chloride (zncl2) is soluble in water. The chemical equation for this reaction is given by: Zncl 2.2h 2 o → zncl(oh) + h 2 o. Zinc Chloride Dissolved In Water.
From www.chegg.com
Solved Suppose 2.07 g of zinc chloride is dissolved in Zinc Chloride Dissolved In Water to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. — yes, zinc chloride (zncl2) is soluble in water. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. With excess water, zinc. Zinc Chloride Dissolved In Water.
From www.sunshinetrading.co
Zinc Chloride Sunshine Trading Company Zinc Chloride Dissolved In Water With excess water, zinc oxychlorides are formed. 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. zinc chloride is very soluble in water & will usually form clear solution. 1 2 it readily dissolves in water to form a clear, colorless solution. As potassium chloride (kcl) dissolves. Zinc Chloride Dissolved In Water.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Photographs The Art of Zinc Chloride Dissolved In Water The chemical equation for this reaction is given by: zinc chloride is very soluble in water & will usually form clear solution. — in this video we will describe the equation zncl2 + h2o and write what. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. 1 2. Zinc Chloride Dissolved In Water.
From www.carolina.com
Zinc Chloride, 1.0 M Aqueous, Laboratory Grade, 500 mL Carolina Biological Supply Zinc Chloride Dissolved In Water 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. The chemical equation for this reaction is given by: Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl As potassium chloride (kcl) dissolves in water, the ions are hydrated. The polar water molecules are. 1 2. Zinc Chloride Dissolved In Water.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Chloride Dissolved In Water — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. zinc chloride is very soluble in water & will usually form clear solution. 1 2 it readily dissolves in water to form a clear, colorless solution. The chemical equation for this reaction is given by: The polar water molecules are.. Zinc Chloride Dissolved In Water.
From www.chegg.com
Solved 200.5 g of zinc chloride ZnCl2 was dissolved in Zinc Chloride Dissolved In Water With excess water, zinc oxychlorides are formed. — when heated, the hydrated form of zinc chloride loses water and small quantities of zncl(oh) are obtained. — in this video we will describe the equation zncl2 + h2o and write what. The polar water molecules are. zinc chloride is very soluble in water & will usually form clear. Zinc Chloride Dissolved In Water.
From www.youtube.com
Double displacement of ZnSO4 + BaCl2 Zinc sulphate + Barium chloride Precipitation reaction Zinc Chloride Dissolved In Water The chemical equation for this reaction is given by: — in this video we will describe the equation zncl2 + h2o and write what. Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl With excess water, zinc oxychlorides are formed. — yes, zinc chloride (zncl2) is soluble in water. 1 2 it readily dissolves in. Zinc Chloride Dissolved In Water.
From www.chegg.com
Solved and The Suppose 20.2 g of zinc chloride is dissolved Zinc Chloride Dissolved In Water The chemical equation for this reaction is given by: 133 rows — — in general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if. — yes, zinc chloride (zncl2) is soluble in water. With excess water, zinc oxychlorides are formed. — in this video we will describe the equation zncl2 + h2o. Zinc Chloride Dissolved In Water.
From www.pw.live
Zinc Chloride Formula Zinc Chloride Dissolved In Water With excess water, zinc oxychlorides are formed. — yes, zinc chloride (zncl2) is soluble in water. The chemical equation for this reaction is given by: to prepare 1000 ml of a 0.1 mol/l solution of zinc chloride we have to dissolve 13.628 g of zncl2 (100 % purity) in deionized or. As potassium chloride (kcl) dissolves in water,. Zinc Chloride Dissolved In Water.
From www.laballey.com
Buy Zinc Chloride, 1M Solution 35+ Bulk Sizes Zinc Chloride Dissolved In Water The chemical equation for this reaction is given by: Zncl 2.2h 2 o → zncl(oh) + h 2 o + hcl — in this video we will describe the equation zncl2 + h2o and write what. The polar water molecules are. — yes, zinc chloride (zncl2) is soluble in water. With excess water, zinc oxychlorides are formed. 1. Zinc Chloride Dissolved In Water.