Copper Chloride Polar Or Nonpolar . Determine if a molecule is polar or. Explain how polar compounds differ from nonpolar compounds. When the difference is very small or zero, the bond is covalent and nonpolar. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. When it is large, the bond is polar covalent or ionic. Every sufficiently asymmetric molecule will be polar, but some more than others. From the lewis structure, and using vsepr theory, we determine that the co 2. Each of the bonds is polar, but the molecule as a whole is nonpolar. Covalent bonds can be broken. In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be. The polarity of molecules is related to the.
from www.slideserve.com
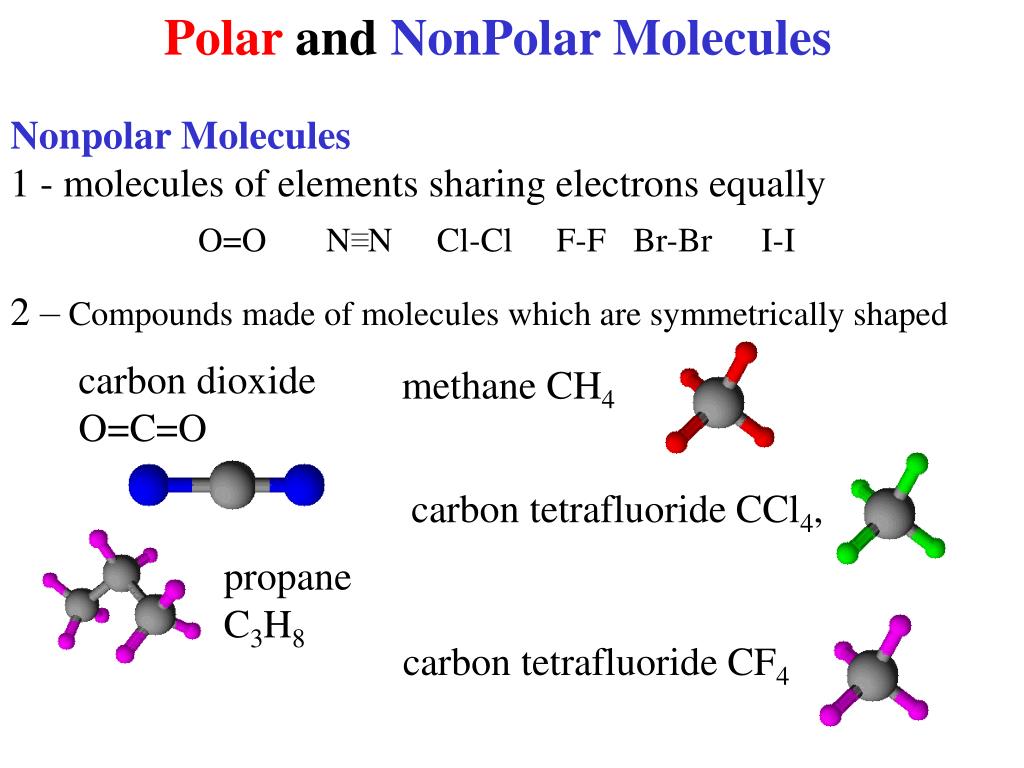
The polarity of molecules is related to the. From the lewis structure, and using vsepr theory, we determine that the co 2. When it is large, the bond is polar covalent or ionic. Each of the bonds is polar, but the molecule as a whole is nonpolar. Determine if a molecule is polar or. Covalent bonds can be broken. Explain how polar compounds differ from nonpolar compounds. Every sufficiently asymmetric molecule will be polar, but some more than others. When the difference is very small or zero, the bond is covalent and nonpolar. In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be.
PPT Polar and NonPolar Molecules PowerPoint Presentation, free
Copper Chloride Polar Or Nonpolar Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. When the difference is very small or zero, the bond is covalent and nonpolar. Every sufficiently asymmetric molecule will be polar, but some more than others. Covalent bonds can be broken. The polarity of molecules is related to the. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Determine if a molecule is polar or. From the lewis structure, and using vsepr theory, we determine that the co 2. When it is large, the bond is polar covalent or ionic. In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be. Explain how polar compounds differ from nonpolar compounds. Each of the bonds is polar, but the molecule as a whole is nonpolar.
From www.youtube.com
Is CaCl2 Polar or Nonpolar? (Calcium Chloride, Ionic) YouTube Copper Chloride Polar Or Nonpolar The polarity of molecules is related to the. Covalent bonds can be broken. Explain how polar compounds differ from nonpolar compounds. From the lewis structure, and using vsepr theory, we determine that the co 2. When the difference is very small or zero, the bond is covalent and nonpolar. Covalent bonds can be nonpolar or polar, depending on the electronegativities. Copper Chloride Polar Or Nonpolar.
From classlibraryfruehauf.z19.web.core.windows.net
Polar Or Nonpolar Chart Copper Chloride Polar Or Nonpolar Covalent bonds can be broken. From the lewis structure, and using vsepr theory, we determine that the co 2. The polarity of molecules is related to the. When the difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent or ionic. Covalent bonds can be nonpolar or polar, depending. Copper Chloride Polar Or Nonpolar.
From www.youtube.com
NaCl Polar or Nonpolar (Sodium Chloride) YouTube Copper Chloride Polar Or Nonpolar When it is large, the bond is polar covalent or ionic. Covalent bonds can be broken. When the difference is very small or zero, the bond is covalent and nonpolar. From the lewis structure, and using vsepr theory, we determine that the co 2. Every sufficiently asymmetric molecule will be polar, but some more than others. In the same way. Copper Chloride Polar Or Nonpolar.
From piyantopzz.blogspot.com
Ch4 Bond Type Polar Or Nonpolar Is Ch4 Methane Polar Or Nonpolar Copper Chloride Polar Or Nonpolar Every sufficiently asymmetric molecule will be polar, but some more than others. The polarity of molecules is related to the. From the lewis structure, and using vsepr theory, we determine that the co 2. Determine if a molecule is polar or. Explain how polar compounds differ from nonpolar compounds. When the difference is very small or zero, the bond is. Copper Chloride Polar Or Nonpolar.
From tiatuwagner.blogspot.com
Hcl Polar or Nonpolar TiatuWagner Copper Chloride Polar Or Nonpolar The polarity of molecules is related to the. When it is large, the bond is polar covalent or ionic. Every sufficiently asymmetric molecule will be polar, but some more than others. Explain how polar compounds differ from nonpolar compounds. When the difference is very small or zero, the bond is covalent and nonpolar. From the lewis structure, and using vsepr. Copper Chloride Polar Or Nonpolar.
From techiescientist.com
Is BeCl2 Polar or Nonpolar? Techiescientist Copper Chloride Polar Or Nonpolar The polarity of molecules is related to the. When the difference is very small or zero, the bond is covalent and nonpolar. Explain how polar compounds differ from nonpolar compounds. Every sufficiently asymmetric molecule will be polar, but some more than others. Determine if a molecule is polar or. When it is large, the bond is polar covalent or ionic.. Copper Chloride Polar Or Nonpolar.
From www.slideserve.com
PPT Polar and NonPolar Molecules PowerPoint Presentation, free Copper Chloride Polar Or Nonpolar When the difference is very small or zero, the bond is covalent and nonpolar. Explain how polar compounds differ from nonpolar compounds. Determine if a molecule is polar or. When it is large, the bond is polar covalent or ionic. Covalent bonds can be broken. In the same way that polar molecules are attracted to the positive and negative plates. Copper Chloride Polar Or Nonpolar.
From www.animalia-life.club
Copper Chloride Copper Chloride Polar Or Nonpolar Each of the bonds is polar, but the molecule as a whole is nonpolar. When the difference is very small or zero, the bond is covalent and nonpolar. The polarity of molecules is related to the. Every sufficiently asymmetric molecule will be polar, but some more than others. When it is large, the bond is polar covalent or ionic. From. Copper Chloride Polar Or Nonpolar.
From lucia-has-beasley.blogspot.com
F2 Polar or Nonpolar Atom Closest to Negative Side LuciahasBeasley Copper Chloride Polar Or Nonpolar Determine if a molecule is polar or. The polarity of molecules is related to the. Every sufficiently asymmetric molecule will be polar, but some more than others. From the lewis structure, and using vsepr theory, we determine that the co 2. Each of the bonds is polar, but the molecule as a whole is nonpolar. Covalent bonds can be broken.. Copper Chloride Polar Or Nonpolar.
From studylib.net
Polar and Nonpolar Molecules Copper Chloride Polar Or Nonpolar From the lewis structure, and using vsepr theory, we determine that the co 2. The polarity of molecules is related to the. Covalent bonds can be broken. Each of the bonds is polar, but the molecule as a whole is nonpolar. In the same way that polar molecules are attracted to the positive and negative plates in an electric field,. Copper Chloride Polar Or Nonpolar.
From slideplayer.com
WARM UP How do you determine if a bond is ionic, polar covalent, or Copper Chloride Polar Or Nonpolar When it is large, the bond is polar covalent or ionic. When the difference is very small or zero, the bond is covalent and nonpolar. Every sufficiently asymmetric molecule will be polar, but some more than others. Determine if a molecule is polar or. Explain how polar compounds differ from nonpolar compounds. From the lewis structure, and using vsepr theory,. Copper Chloride Polar Or Nonpolar.
From www.youtube.com
Is CCl4 Polar or Nonpolar? (Carbon Tetrachloride) YouTube Copper Chloride Polar Or Nonpolar In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be. From the lewis structure, and using vsepr theory, we determine that the co 2. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken. Explain how polar compounds. Copper Chloride Polar Or Nonpolar.
From quizlet.com
Is hydrogen chloride, \ce{HCl}, polar or nonpolar? Quizlet Copper Chloride Polar Or Nonpolar Explain how polar compounds differ from nonpolar compounds. Covalent bonds can be broken. Every sufficiently asymmetric molecule will be polar, but some more than others. When the difference is very small or zero, the bond is covalent and nonpolar. Each of the bonds is polar, but the molecule as a whole is nonpolar. From the lewis structure, and using vsepr. Copper Chloride Polar Or Nonpolar.
From draw-hub.blogspot.com
Do Polar Or Nonpolar Molecules Dissolve In Water Drawhub Copper Chloride Polar Or Nonpolar Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. When the difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent or ionic. The polarity of molecules is related to the. Each of the bonds is polar, but the molecule as a whole. Copper Chloride Polar Or Nonpolar.
From www.pinterest.co.uk
Comparision of Polar, NonPolar and Ionic Bonds Surfguppy Chemistry Copper Chloride Polar Or Nonpolar When it is large, the bond is polar covalent or ionic. The polarity of molecules is related to the. Explain how polar compounds differ from nonpolar compounds. Every sufficiently asymmetric molecule will be polar, but some more than others. Each of the bonds is polar, but the molecule as a whole is nonpolar. Determine if a molecule is polar or.. Copper Chloride Polar Or Nonpolar.
From byjus.com
Is ICl2 nonpolar or polar? Copper Chloride Polar Or Nonpolar Explain how polar compounds differ from nonpolar compounds. When the difference is very small or zero, the bond is covalent and nonpolar. Each of the bonds is polar, but the molecule as a whole is nonpolar. The polarity of molecules is related to the. Every sufficiently asymmetric molecule will be polar, but some more than others. When it is large,. Copper Chloride Polar Or Nonpolar.
From www.youtube.com
Is NaCl (Sodium chloride) Ionic or Covalent? YouTube Copper Chloride Polar Or Nonpolar When the difference is very small or zero, the bond is covalent and nonpolar. Each of the bonds is polar, but the molecule as a whole is nonpolar. Determine if a molecule is polar or. When it is large, the bond is polar covalent or ionic. In the same way that polar molecules are attracted to the positive and negative. Copper Chloride Polar Or Nonpolar.
From www.slideserve.com
PPT Electronegativity & Polarity PowerPoint Presentation, free Copper Chloride Polar Or Nonpolar Every sufficiently asymmetric molecule will be polar, but some more than others. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Determine if a molecule is polar or. The polarity of molecules is related to the. Each of the bonds is polar, but the molecule as a whole is nonpolar. Covalent bonds can be. Copper Chloride Polar Or Nonpolar.
From topblogtenz.com
Is Diethyl Ether Polar or Nonpolar? (Polarity of diethyl ether) Copper Chloride Polar Or Nonpolar From the lewis structure, and using vsepr theory, we determine that the co 2. Explain how polar compounds differ from nonpolar compounds. Covalent bonds can be broken. Every sufficiently asymmetric molecule will be polar, but some more than others. When the difference is very small or zero, the bond is covalent and nonpolar. Determine if a molecule is polar or.. Copper Chloride Polar Or Nonpolar.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Copper Chloride Polar Or Nonpolar Determine if a molecule is polar or. From the lewis structure, and using vsepr theory, we determine that the co 2. In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be. Each of the bonds is polar, but the molecule as a whole is nonpolar. Every sufficiently asymmetric. Copper Chloride Polar Or Nonpolar.
From www.youtube.com
Is NaCl Polar or Nonpolar? YouTube Copper Chloride Polar Or Nonpolar When it is large, the bond is polar covalent or ionic. The polarity of molecules is related to the. Every sufficiently asymmetric molecule will be polar, but some more than others. Covalent bonds can be broken. From the lewis structure, and using vsepr theory, we determine that the co 2. Determine if a molecule is polar or. Covalent bonds can. Copper Chloride Polar Or Nonpolar.
From democracyunlimited.web.fc2.com
is sodium chloride polar or nonpolar Copper Chloride Polar Or Nonpolar In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be. Every sufficiently asymmetric molecule will be polar, but some more than others. Determine if a molecule is polar or. Each of the bonds is polar, but the molecule as a whole is nonpolar. From the lewis structure, and. Copper Chloride Polar Or Nonpolar.
From www.youtube.com
Polar vs Nonpolar molecules How to tell? [GCE A Level Chemistry] YouTube Copper Chloride Polar Or Nonpolar Each of the bonds is polar, but the molecule as a whole is nonpolar. When the difference is very small or zero, the bond is covalent and nonpolar. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. From the lewis structure, and using vsepr theory, we determine that the co 2. When it is. Copper Chloride Polar Or Nonpolar.
From 2012books.lardbucket.org
Polar Covalent Bonds Copper Chloride Polar Or Nonpolar From the lewis structure, and using vsepr theory, we determine that the co 2. Determine if a molecule is polar or. Explain how polar compounds differ from nonpolar compounds. When it is large, the bond is polar covalent or ionic. Covalent bonds can be broken. Each of the bonds is polar, but the molecule as a whole is nonpolar. Every. Copper Chloride Polar Or Nonpolar.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts Copper Chloride Polar Or Nonpolar Every sufficiently asymmetric molecule will be polar, but some more than others. From the lewis structure, and using vsepr theory, we determine that the co 2. The polarity of molecules is related to the. When the difference is very small or zero, the bond is covalent and nonpolar. Determine if a molecule is polar or. Explain how polar compounds differ. Copper Chloride Polar Or Nonpolar.
From www.reddit.com
I made some copper chloride recently and it turned out really beautiful Copper Chloride Polar Or Nonpolar When the difference is very small or zero, the bond is covalent and nonpolar. The polarity of molecules is related to the. From the lewis structure, and using vsepr theory, we determine that the co 2. When it is large, the bond is polar covalent or ionic. Determine if a molecule is polar or. Covalent bonds can be broken. In. Copper Chloride Polar Or Nonpolar.
From www.science-revision.co.uk
Electronegativity and polar bonds Copper Chloride Polar Or Nonpolar Determine if a molecule is polar or. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken. When the difference is very small or zero, the bond is covalent and nonpolar. Each of the bonds is polar, but the molecule as a whole is nonpolar. The polarity of molecules is. Copper Chloride Polar Or Nonpolar.
From www.numerade.com
SOLVED Classify each of the solutes . polar or nonpolar (yes/no Copper Chloride Polar Or Nonpolar Determine if a molecule is polar or. Each of the bonds is polar, but the molecule as a whole is nonpolar. In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be. From the lewis structure, and using vsepr theory, we determine that the co 2. Covalent bonds can. Copper Chloride Polar Or Nonpolar.
From www.numerade.com
SOLVED Are the following compounds polar or nonpolar? Carbon dioxide Copper Chloride Polar Or Nonpolar The polarity of molecules is related to the. Covalent bonds can be broken. Determine if a molecule is polar or. Explain how polar compounds differ from nonpolar compounds. When it is large, the bond is polar covalent or ionic. When the difference is very small or zero, the bond is covalent and nonpolar. Each of the bonds is polar, but. Copper Chloride Polar Or Nonpolar.
From general.chemistrysteps.com
CH2Cl2 Polar or Nonpolar Chemistry Steps Copper Chloride Polar Or Nonpolar Determine if a molecule is polar or. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. From the lewis structure, and using vsepr theory, we determine that the co 2. The polarity of molecules is related to the. Each of the bonds is polar, but the molecule as a whole is nonpolar. When the. Copper Chloride Polar Or Nonpolar.
From simp-link.com
Difference between polar and nonpolar examples Copper Chloride Polar Or Nonpolar Determine if a molecule is polar or. Each of the bonds is polar, but the molecule as a whole is nonpolar. When the difference is very small or zero, the bond is covalent and nonpolar. The polarity of molecules is related to the. From the lewis structure, and using vsepr theory, we determine that the co 2. When it is. Copper Chloride Polar Or Nonpolar.
From brainly.in
Sbcl5 polar or nonpolar Brainly.in Copper Chloride Polar Or Nonpolar The polarity of molecules is related to the. Every sufficiently asymmetric molecule will be polar, but some more than others. When it is large, the bond is polar covalent or ionic. Covalent bonds can be broken. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. In the same way that polar molecules are attracted. Copper Chloride Polar Or Nonpolar.
From www.pinterest.ph
BeCl2 polar or nonpolar molecule Polar, Science education, Education Copper Chloride Polar Or Nonpolar When the difference is very small or zero, the bond is covalent and nonpolar. In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken. From the lewis structure,. Copper Chloride Polar Or Nonpolar.
From bertigamas.github.io
Sf6 Polar Atau Nonpolar Brain Copper Chloride Polar Or Nonpolar In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be. Every sufficiently asymmetric molecule will be polar, but some more than others. Determine if a molecule is polar or. From the lewis structure, and using vsepr theory, we determine that the co 2. Explain how polar compounds differ. Copper Chloride Polar Or Nonpolar.
From www.sustarfeed.com
China Tribasic Copper Chloride TBCC Copper Trihydroxyl Chloride Copper Copper Chloride Polar Or Nonpolar Every sufficiently asymmetric molecule will be polar, but some more than others. Determine if a molecule is polar or. From the lewis structure, and using vsepr theory, we determine that the co 2. Covalent bonds can be broken. The polarity of molecules is related to the. When the difference is very small or zero, the bond is covalent and nonpolar.. Copper Chloride Polar Or Nonpolar.