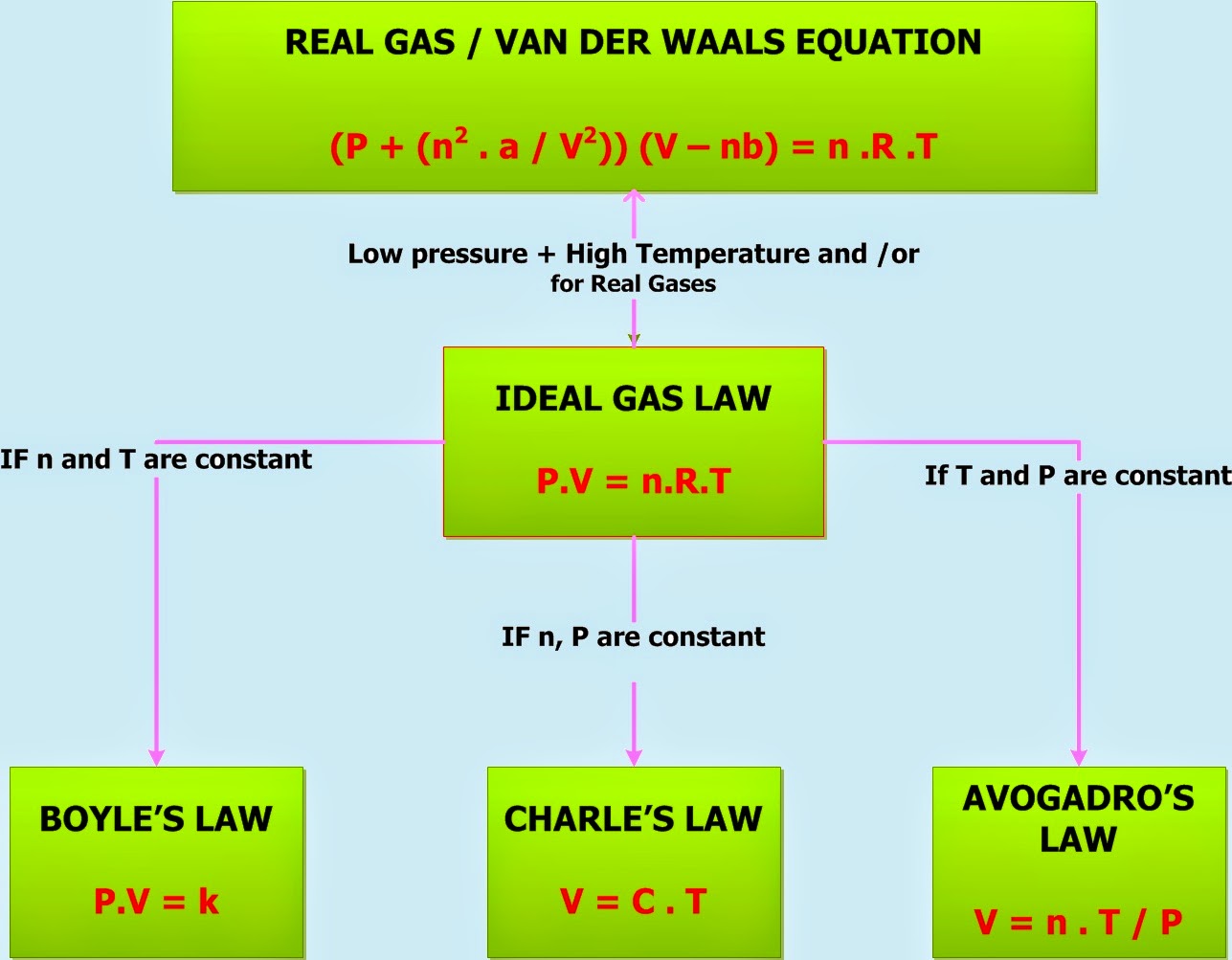
Gas Laws Example Problems . For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. (p 1 v 1) / t 1 = (p 2 v 2) / t 2. The following practice problems are to master to topics on the ideal gas laws: Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. As temperature of a gas increases,. The form of the combined gas law most often used is this: This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas in a system when the pressure, volume, and. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. What is r called (a letter is. Kmt & gas laws menu. What is the value of and units on r? Return to kmt & gas laws menu.
from chem-net.blogspot.com
(p 1 v 1) / t 1 = (p 2 v 2) / t 2. This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas in a system when the pressure, volume, and. What is the value of and units on r? For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. What is r called (a letter is. Return to kmt & gas laws menu. The form of the combined gas law most often used is this: As temperature of a gas increases,. Sometimes leaving a bicycle in the sun on a hot day will cause a blowout.
Gas Laws Ideal Gas Law Chemistry Net
Gas Laws Example Problems For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. Return to kmt & gas laws menu. Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. The following practice problems are to master to topics on the ideal gas laws: As temperature of a gas increases,. Kmt & gas laws menu. (p 1 v 1) / t 1 = (p 2 v 2) / t 2. For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. The form of the combined gas law most often used is this: What is r called (a letter is. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas in a system when the pressure, volume, and. What is the value of and units on r?
From sciencenotes.org
Charles's Law Definition, Formula, Examples Gas Laws Example Problems What is the value of and units on r? (p 1 v 1) / t 1 = (p 2 v 2) / t 2. This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas in a system when the pressure, volume, and. For example, in the boyle’s. Gas Laws Example Problems.
From www.showme.com
Charles' Law example problem Science, Chemistry, Gases ShowMe Gas Laws Example Problems The form of the combined gas law most often used is this: Kmt & gas laws menu. What is the value of and units on r? Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. Return to kmt & gas laws menu. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4,. Gas Laws Example Problems.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Gas Laws Example Problems What is the value of and units on r? Return to kmt & gas laws menu. The following practice problems are to master to topics on the ideal gas laws: Kmt & gas laws menu. What is r called (a letter is. As temperature of a gas increases,. This ideal gas law example problem shows the steps needed to use. Gas Laws Example Problems.
From www.showme.com
Ideal gas law example Science, Chemistry, Gases ShowMe Gas Laws Example Problems What is the value of and units on r? As temperature of a gas increases,. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. The form. Gas Laws Example Problems.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Gas Laws Example Problems Return to kmt & gas laws menu. Kmt & gas laws menu. This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas in a system when the pressure, volume, and. Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. What is. Gas Laws Example Problems.
From www.showme.com
Combined Gas Law Problem Fundamental JMC SP2017 Gas Laws Gas Laws Example Problems What is the value of and units on r? As temperature of a gas increases,. What is r called (a letter is. (p 1 v 1) / t 1 = (p 2 v 2) / t 2. Kmt & gas laws menu. Return to kmt & gas laws menu. The form of the combined gas law most often used is. Gas Laws Example Problems.
From heretup949.weebly.com
Avogadro's Law heretup Gas Laws Example Problems Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. As temperature of a gas increases,. For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. Kmt & gas laws menu. Return to kmt & gas laws. Gas Laws Example Problems.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Gas Laws Example Problems (p 1 v 1) / t 1 = (p 2 v 2) / t 2. The form of the combined gas law most often used is this: Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and. Gas Laws Example Problems.
From printablelibcoburg.z13.web.core.windows.net
Ideal Gas Law Problem Examples Gas Laws Example Problems Kmt & gas laws menu. (p 1 v 1) / t 1 = (p 2 v 2) / t 2. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. For example, in the boyle’s law, we study a constant amount of. Gas Laws Example Problems.
From sciencenotes.org
Boyle's Law Definition, Formula, Example Gas Laws Example Problems (p 1 v 1) / t 1 = (p 2 v 2) / t 2. What is the value of and units on r? For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. A sample of gas isolated from unrefined petroleum. Gas Laws Example Problems.
From www.slideserve.com
PPT Properties of Gases & Gas Laws PowerPoint Presentation, free Gas Laws Example Problems What is the value of and units on r? The form of the combined gas law most often used is this: (p 1 v 1) / t 1 = (p 2 v 2) / t 2. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at. Gas Laws Example Problems.
From www.showme.com
ShowMe derivation of combined gas law Gas Laws Example Problems Kmt & gas laws menu. The following practice problems are to master to topics on the ideal gas laws: The form of the combined gas law most often used is this: Return to kmt & gas laws menu. As temperature of a gas increases,. This ideal gas law example problem shows the steps needed to use the ideal gas law. Gas Laws Example Problems.
From www.youtube.com
Solving Combined Gas Law Problems Charles' Law, Boyle's Law, Lussac's Gas Laws Example Problems The form of the combined gas law most often used is this: As temperature of a gas increases,. Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas in a system when. Gas Laws Example Problems.
From www.slideshare.net
Notes gas laws Gas Laws Example Problems The following practice problems are to master to topics on the ideal gas laws: What is the value of and units on r? Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. What is r called (a letter is. Kmt & gas laws menu. (p 1 v 1) / t 1 = (p 2. Gas Laws Example Problems.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID3252378 Gas Laws Example Problems The form of the combined gas law most often used is this: As temperature of a gas increases,. Return to kmt & gas laws menu. What is the value of and units on r? Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. (p 1 v 1) / t 1 = (p 2 v. Gas Laws Example Problems.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Gas Laws Example Problems (p 1 v 1) / t 1 = (p 2 v 2) / t 2. What is the value of and units on r? The following practice problems are to master to topics on the ideal gas laws: Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. For example, in the boyle’s law, we. Gas Laws Example Problems.
From www.youtube.com
Density with the Ideal Gas Law Chemistry Sample Problem YouTube Gas Laws Example Problems The following practice problems are to master to topics on the ideal gas laws: Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. What is r called (a letter is. The form of the combined gas law most often used is this: As temperature of a gas increases,. What is the value of and. Gas Laws Example Problems.
From www.studypool.com
SOLUTION Ideal gas law worksheet 2 answer Studypool Gas Laws Example Problems The form of the combined gas law most often used is this: For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. Kmt & gas laws menu. As temperature of a gas increases,. The following practice problems are to master to topics. Gas Laws Example Problems.
From www.youtube.com
Ideal Gas Law Example Problems YouTube Gas Laws Example Problems Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. What is r called (a letter is. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. Kmt & gas laws menu. The following practice problems are. Gas Laws Example Problems.
From www.youtube.com
Boyle's Law Sample Problem YouTube Gas Laws Example Problems Kmt & gas laws menu. This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas in a system when the pressure, volume, and. Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. For example, in the boyle’s law, we study a. Gas Laws Example Problems.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Gas Laws Example Problems Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. (p 1 v 1) / t 1 = (p 2 v 2) / t 2. As temperature. Gas Laws Example Problems.
From www.slideserve.com
PPT Gas Laws and Nature of Gases PowerPoint Presentation, free Gas Laws Example Problems This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas in a system when the pressure, volume, and. (p 1 v 1) / t 1 = (p 2 v 2) / t 2. What is r called (a letter is. For example, in the boyle’s law, we. Gas Laws Example Problems.
From www.slideserve.com
PPT Gas Law Notes Chemistry Semester II PowerPoint Presentation, free Gas Laws Example Problems The following practice problems are to master to topics on the ideal gas laws: For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. This ideal gas law example problem shows the steps needed to use the ideal gas law equation to. Gas Laws Example Problems.
From www.slideshare.net
Gas Law Sample Problems Gas Laws Example Problems What is r called (a letter is. Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. The following practice problems are to master to topics on the ideal gas laws: What is the value of and units on r? Kmt & gas laws menu. Return to kmt & gas laws menu. For example, in. Gas Laws Example Problems.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Gas Laws Example Problems What is the value of and units on r? The form of the combined gas law most often used is this: A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. What is r called (a letter is. This ideal gas law. Gas Laws Example Problems.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Gas Laws Example Problems For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. As temperature of a gas increases,. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total. Gas Laws Example Problems.
From www.slideshare.net
Ideal Gas Law Problems Gas Laws Example Problems What is r called (a letter is. Kmt & gas laws menu. For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. The following practice problems are to master to topics on the ideal gas laws: A sample of gas isolated from. Gas Laws Example Problems.
From www.nagwa.com
Question Video Using GayLussac’s Law to Find the Pressure of a Gas Gas Laws Example Problems A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. What is the value of and units on r? This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas. Gas Laws Example Problems.
From www.youtube.com
Ideal Gas Law Practice Problems YouTube Gas Laws Example Problems A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. What is the value of and units on r? What is r called (a letter is. The following practice problems are to master to topics on the ideal gas laws: For example,. Gas Laws Example Problems.
From www.youtube.com
Combined Gas Law Problems YouTube Gas Laws Example Problems This ideal gas law example problem shows the steps needed to use the ideal gas law equation to determine the amount of gas in a system when the pressure, volume, and. What is the value of and units on r? A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c. Gas Laws Example Problems.
From www.youtube.com
Gas Law Practice Problems Boyle's Law, Charles' Law, Gay Lussac's Gas Laws Example Problems What is r called (a letter is. The following practice problems are to master to topics on the ideal gas laws: Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as. Gas Laws Example Problems.
From www.slideshare.net
Ideal Gas Law Problems Gas Laws Example Problems Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. Kmt & gas laws menu. What is the value of and units on r? The form of. Gas Laws Example Problems.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Gas Laws Example Problems A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of. (p 1 v 1) / t 1 = (p 2 v 2) / t 2. Kmt & gas laws menu. The following practice problems are to master to topics on the ideal. Gas Laws Example Problems.
From asiasupergrid.com
Ideal Gas Law Equation Example Gas Laws Example Problems Return to kmt & gas laws menu. The form of the combined gas law most often used is this: As temperature of a gas increases,. (p 1 v 1) / t 1 = (p 2 v 2) / t 2. A sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c. Gas Laws Example Problems.
From www.youtube.com
IDEAL GAS LAW PRACTICE PROBLEMS How to Solve Ideal Gas Law Problems Gas Laws Example Problems What is the value of and units on r? Return to kmt & gas laws menu. For example, in the boyle’s law, we study a constant amount of gas at a constant temperature and find that the pressure increase as the volume is decreased. (p 1 v 1) / t 1 = (p 2 v 2) / t 2. What. Gas Laws Example Problems.