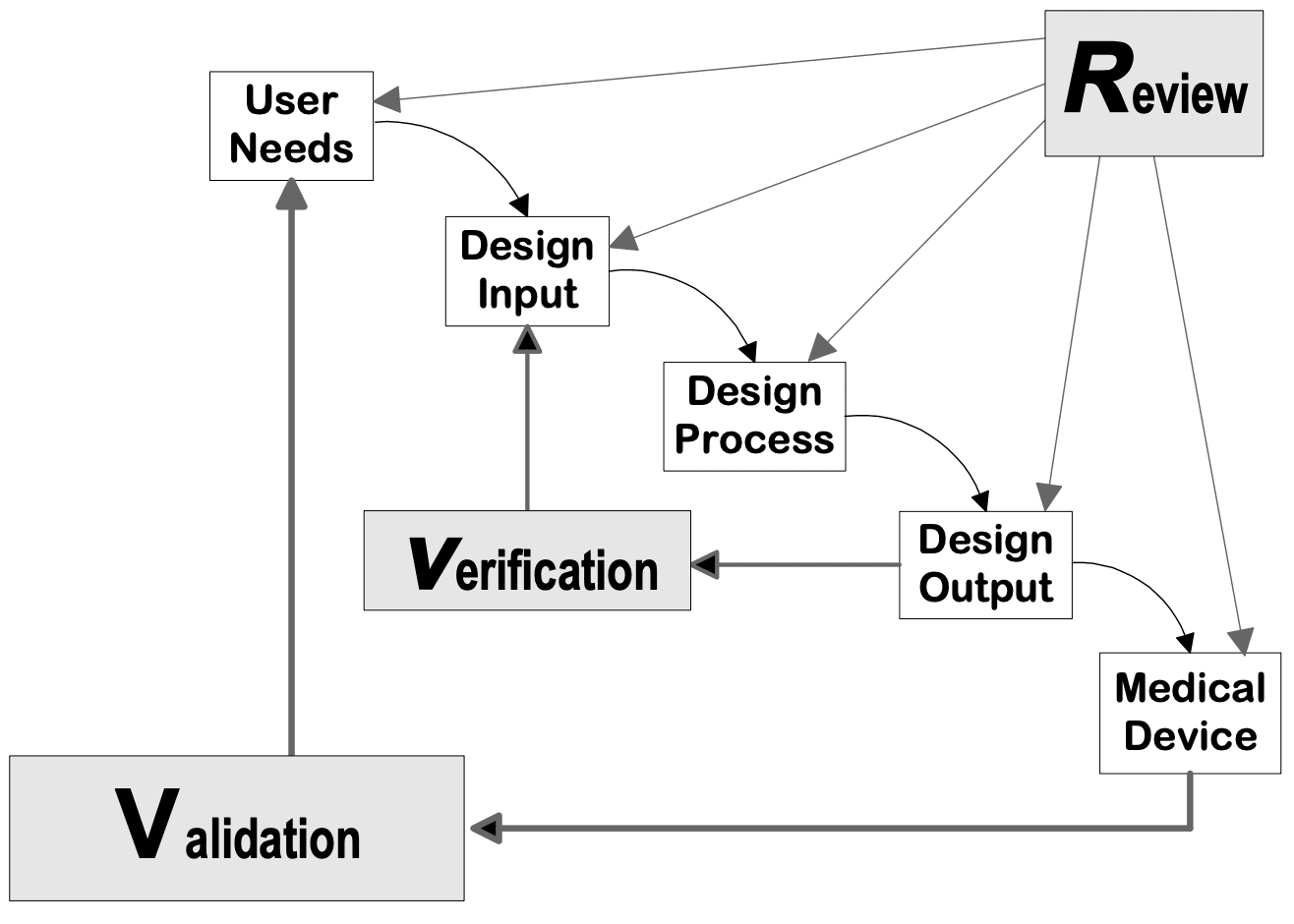
Medical Device Design Outputs . This guidance document describes different study design principles relevant to the development of medical device clinical studies. Safe medical device act of 1990 authorized fda to add design controls to the current good. Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Design outputs describe all the components, parts, and pieces that go into your medical device.
from www.kolabtree.com
This guidance document describes different study design principles relevant to the development of medical device clinical studies. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Design outputs describe all the components, parts, and pieces that go into your medical device. Safe medical device act of 1990 authorized fda to add design controls to the current good.
A guide to FDA Design Controls for your medical device
Medical Device Design Outputs This guidance document describes different study design principles relevant to the development of medical device clinical studies. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Safe medical device act of 1990 authorized fda to add design controls to the current good. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. This guidance document describes different study design principles relevant to the development of medical device clinical studies. Design outputs describe all the components, parts, and pieces that go into your medical device. Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into.
From medicaldevicehq.com
The Perfect Project Process Medical Device Product Development Medical Device Design Outputs This guidance document describes different study design principles relevant to the development of medical device clinical studies. Design outputs describe all the components, parts, and pieces that go into your medical device. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right. Medical Device Design Outputs.
From www.springboard.pro
Design controls in medical device development Ensuring safety and Medical Device Design Outputs The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design. Medical Device Design Outputs.
From www.baatmedical.com
Medical Device Development Design Control Medical Devices Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Safe medical device act of 1990 authorized fda to add design controls to the current good. This guidance document describes different study design principles relevant. Medical Device Design Outputs.
From www.qmdocs.com
Medical Device Design Outputs Medical Device Design Outputs Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. Design outputs describe all the components, parts, and pieces that go into your medical device. This guidance document describes different study design principles relevant to the development of medical device clinical studies. If you are thorough with defining and. Medical Device Design Outputs.
From www.pinterest.com
Browse Our Sample of Medical Device Design And Development Plan Medical Device Design Outputs Design outputs describe all the components, parts, and pieces that go into your medical device. This guidance document describes different study design principles relevant to the development of medical device clinical studies. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Your design outputs are there to describe the actual. Medical Device Design Outputs.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Design outputs describe all the components, parts, and pieces that go into your medical device. Safe medical device act of 1990 authorized fda to add. Medical Device Design Outputs.
From medicaldevicehq.com
Medical device design control terminology Medical Device HQ 1 Medical Device Design Outputs Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. Design outputs describe all the components, parts, and pieces that go into your medical device. This guidance document describes different study design principles relevant to the development of medical device clinical studies. The guidance applies to the design of. Medical Device Design Outputs.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. The guidance applies to. Medical Device Design Outputs.
From www.healthtard.com
Medical Device Design Control Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Design outputs describe all the components, parts, and pieces that go into your medical device. Your design outputs are there to describe the actual device. Medical Device Design Outputs.
From www.joharidigital.com
Medical Device Design and Development The Ultimate Guide from scratch Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Design outputs describe all the components, parts, and pieces that go into your medical device. Safe medical device act of 1990 authorized fda to add. Medical Device Design Outputs.
From www.kolabtree.com
A guide to FDA Design Controls for your medical device Medical Device Design Outputs Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. This guidance document describes different study design principles relevant to the development of medical device clinical studies. Safe medical device act of 1990 authorized fda to add design controls to the current good. Design outputs describe all the components,. Medical Device Design Outputs.
From www.modernrequirements.com
Facilitate the Medical Device Design Controls Modern Requirements Medical Device Design Outputs Safe medical device act of 1990 authorized fda to add design controls to the current good. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is The guidance applies to the design of medical devices. Medical Device Design Outputs.
From www.researchgate.net
(PDF) Identifying the initial specification of Design Inputs & Outputs Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. This guidance document describes. Medical Device Design Outputs.
From www.proximacro.com
Design Controls Digest 5 Phases of Design Controls You Need to Know Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Safe medical device act of 1990 authorized fda to add design controls to the current good. Your design outputs are there to describe the actual. Medical Device Design Outputs.
From patientguard.com
Medical Device Design and Development Medical Device Design Outputs Safe medical device act of 1990 authorized fda to add design controls to the current good. Design outputs describe all the components, parts, and pieces that go into your medical device. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. If you are thorough with defining and documenting user needs,. Medical Device Design Outputs.
From www.greenlight.guru
The Art of Defining Design Inputs And Design Outputs Medical Device Design Outputs This guidance document describes different study design principles relevant to the development of medical device clinical studies. Design outputs describe all the components, parts, and pieces that go into your medical device. Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. The guidance applies to the design of. Medical Device Design Outputs.
From www.greenlight.guru
Design Controls For Medical Device Companies [Guide] Medical Device Design Outputs This guidance document describes different study design principles relevant to the development of medical device clinical studies. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will. Medical Device Design Outputs.
From www.orielstat.com
Basics of Medical Device Design Controls What, Why, and How Oriel Medical Device Design Outputs Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Design outputs describe all the components, parts, and pieces that go into your medical device. This guidance document describes different. Medical Device Design Outputs.
From www.greenlight.guru
The Art of Defining Design Inputs And Design Outputs Medical Device Design Outputs Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. Design outputs describe all the components, parts, and pieces that go into your medical device. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Safe medical device act of. Medical Device Design Outputs.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Design outputs describe all the components, parts, and pieces that go into your medical device. This guidance document describes different study design principles relevant to. Medical Device Design Outputs.
From www.orielstat.com
Basics of Medical Device Design Controls What, Why, and How Oriel Medical Device Design Outputs Design outputs describe all the components, parts, and pieces that go into your medical device. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is The guidance applies to the design of medical devices as. Medical Device Design Outputs.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Medical Device Design Outputs The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Design outputs describe all the components,. Medical Device Design Outputs.
From www.johner-institut.de
Design Output Inhalt und regulatorische Anfoderungen Medical Device Design Outputs This guidance document describes different study design principles relevant to the development of medical device clinical studies. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will. Medical Device Design Outputs.
From operonstrategist.com
FDA Design Control The Ultimate Guide For Medical Device Companies Medical Device Design Outputs The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Design outputs describe all the components,. Medical Device Design Outputs.
From www.qmdocs.com
Medical Device Design Control SOP Medical Device Design Outputs Design outputs describe all the components, parts, and pieces that go into your medical device. Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will. Medical Device Design Outputs.
From sunstonepilot.com
What Are Medical Device Design Controls? Sunstone Pilot, Inc. Medical Device Design Outputs Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. Safe medical device act of 1990 authorized fda to add design controls to the current good. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Design outputs describe all. Medical Device Design Outputs.
From vem-medical.com
Medical Device Design Development Medical Device Design Outputs Design outputs describe all the components, parts, and pieces that go into your medical device. Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. Safe medical device act of 1990 authorized fda to add design controls to the current good. If you are thorough with defining and documenting. Medical Device Design Outputs.
From www.youtube.com
Design Control for Medical Devices Online introductory course YouTube Medical Device Design Outputs This guidance document describes different study design principles relevant to the development of medical device clinical studies. The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Design outputs describe all the components, parts, and pieces that go into your medical device. Your design outputs are there to describe the actual. Medical Device Design Outputs.
From www.greenlight.guru
What Device Makers Need To Know About Design Verification and Validation Medical Device Design Outputs Safe medical device act of 1990 authorized fda to add design controls to the current good. This guidance document describes different study design principles relevant to the development of medical device clinical studies. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the. Medical Device Design Outputs.
From www.greenlight.guru
The Art of Defining Design Inputs And Design Outputs Medical Device Design Outputs The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. This guidance document describes different study design principles relevant to the development of medical device clinical studies. Safe medical device act of 1990 authorized fda to add design controls to the current good. Your design outputs are there to describe the. Medical Device Design Outputs.
From templates.rjuuc.edu.np
Regulatory Strategy Template For Medical Devices Medical Device Design Outputs The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is This guidance document describes different study. Medical Device Design Outputs.
From www.elexes.com
Medical Device Design & Development Guide! Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Design outputs describe all the components,. Medical Device Design Outputs.
From synergio.nl
Agile Medical Device Development Do or Don't? Content aspects Medical Device Design Outputs Design outputs describe all the components, parts, and pieces that go into your medical device. Your design outputs are there to describe the actual device and should include the “recipe” or any documentation that goes into. If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will. Medical Device Design Outputs.
From www.joharidigital.com
What is Design History File? Why it is Important for Medical Device Medical Device Design Outputs If you are thorough with defining and documenting user needs, design inputs, design outputs, design verification, design validation, and design reviews, then you will be on the right track towards ensuring your medical device is Design outputs describe all the components, parts, and pieces that go into your medical device. Safe medical device act of 1990 authorized fda to add. Medical Device Design Outputs.
From templates.rjuuc.edu.np
Medical Device Traceability Matrix Template Medical Device Design Outputs The guidance applies to the design of medical devices as well as the design of the associated manufacturing processes. Safe medical device act of 1990 authorized fda to add design controls to the current good. This guidance document describes different study design principles relevant to the development of medical device clinical studies. If you are thorough with defining and documenting. Medical Device Design Outputs.