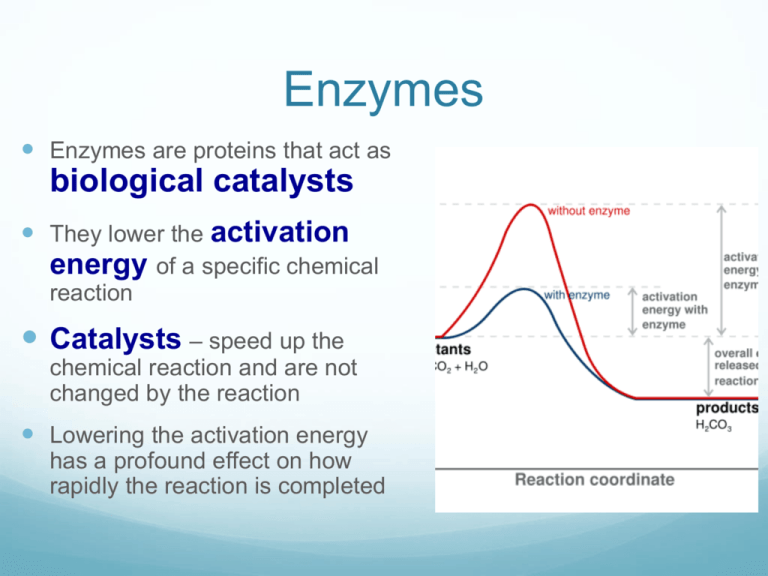
Explain Catalytic Action In Terms Of Activation Energy . This does not change the frequency of collisions. the activation energy is the minimum energy required for a reaction to occur. the activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at a particular concentration, the rate. Decreased activation energy means less energy required to start the reaction. Catalysts increase rate of reaction by providing an alternative pathway, which. This means that the reactant molecules have enough. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. explain catalytic action in terms of activation energy. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. a substance that modifies the transition state to lower the activation energy is termed a catalyst; a catalyst increases the rate of reaction by decreasing the activation energy.
from studylib.net
Decreased activation energy means less energy required to start the reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. This does not change the frequency of collisions. When a catalyst is used at a particular concentration, the rate. the activation energy of a reaction is 19.0 kj/mol. explain catalytic action in terms of activation energy. a catalyst increases the rate of reaction by decreasing the activation energy. the activation energy is the minimum energy required for a reaction to occur. a substance that modifies the transition state to lower the activation energy is termed a catalyst; Catalysts increase rate of reaction by providing an alternative pathway, which.
Enzymes Lower Activation Energy
Explain Catalytic Action In Terms Of Activation Energy explain catalytic action in terms of activation energy. This means that the reactant molecules have enough. the activation energy is the minimum energy required for a reaction to occur. a substance that modifies the transition state to lower the activation energy is termed a catalyst; activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. explain catalytic action in terms of activation energy. When a catalyst is used at a particular concentration, the rate. Decreased activation energy means less energy required to start the reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. Catalysts increase rate of reaction by providing an alternative pathway, which. a catalyst increases the rate of reaction by decreasing the activation energy. This does not change the frequency of collisions. the activation energy of a reaction is 19.0 kj/mol.
From www.researchgate.net
Activation energy for methane combustion with the two catalysts Explain Catalytic Action In Terms Of Activation Energy as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. When a catalyst is used at a particular concentration, the rate. the activation energy is the minimum energy required for a reaction to occur. a catalyst increases the rate of reaction. Explain Catalytic Action In Terms Of Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. a catalyst increases the rate of reaction by decreasing the activation energy. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. This does not change the frequency of collisions. explain catalytic action in terms of activation energy. as. Explain Catalytic Action In Terms Of Activation Energy.
From dxopopljn.blob.core.windows.net
Enzyme Catalyzed Reactions at Richard Fountain blog Explain Catalytic Action In Terms Of Activation Energy Catalysts increase rate of reaction by providing an alternative pathway, which. the activation energy is the minimum energy required for a reaction to occur. This means that the reactant molecules have enough. the activation energy of a reaction is 19.0 kj/mol. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines. Explain Catalytic Action In Terms Of Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. This means that the reactant molecules have enough. This does not change the frequency of collisions. the activation energy is the minimum energy required for a reaction to occur. Catalysts increase rate of reaction by providing an alternative pathway, which. a catalyst increases the rate of reaction by. Explain Catalytic Action In Terms Of Activation Energy.
From sciencenotes.org
What Is Activation Energy? Definition and Examples Explain Catalytic Action In Terms Of Activation Energy Catalysts increase rate of reaction by providing an alternative pathway, which. This means that the reactant molecules have enough. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within. Explain Catalytic Action In Terms Of Activation Energy.
From www.slideserve.com
PPT Enzyme and Catalysis PowerPoint Presentation, free Explain Catalytic Action In Terms Of Activation Energy This does not change the frequency of collisions. When a catalyst is used at a particular concentration, the rate. Decreased activation energy means less energy required to start the reaction. explain catalytic action in terms of activation energy. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. This means that. Explain Catalytic Action In Terms Of Activation Energy.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Explain Catalytic Action In Terms Of Activation Energy as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. This does not change the frequency of collisions. the activation energy of a reaction is 19.0 kj/mol. Catalysts increase rate of reaction by providing an alternative pathway, which. Decreased activation energy means. Explain Catalytic Action In Terms Of Activation Energy.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Explain Catalytic Action In Terms Of Activation Energy explain catalytic action in terms of activation energy. Catalysts increase rate of reaction by providing an alternative pathway, which. This does not change the frequency of collisions. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. a catalyst increases the rate of reaction by decreasing the activation energy. Decreased. Explain Catalytic Action In Terms Of Activation Energy.
From boisestate.pressbooks.pub
12.7 Catalysis General Chemistry 1 & 2 Explain Catalytic Action In Terms Of Activation Energy a substance that modifies the transition state to lower the activation energy is termed a catalyst; the activation energy is the minimum energy required for a reaction to occur. When a catalyst is used at a particular concentration, the rate. Decreased activation energy means less energy required to start the reaction. This means that the reactant molecules have. Explain Catalytic Action In Terms Of Activation Energy.
From thebiologs.blogspot.com
The BioLogs CAPE 1 Enzymes and Enzyme Activity Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. When a catalyst is used at a particular concentration, the rate. This means that the reactant molecules have enough. explain catalytic action in terms of activation energy. a catalyst increases. Explain Catalytic Action In Terms Of Activation Energy.
From www.slideserve.com
PPT Chapter 5 Enzymes PowerPoint Presentation, free download ID Explain Catalytic Action In Terms Of Activation Energy explain catalytic action in terms of activation energy. This means that the reactant molecules have enough. Catalysts increase rate of reaction by providing an alternative pathway, which. Decreased activation energy means less energy required to start the reaction. the activation energy is the minimum energy required for a reaction to occur. the activation energy of a reaction. Explain Catalytic Action In Terms Of Activation Energy.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID1785599 Explain Catalytic Action In Terms Of Activation Energy explain catalytic action in terms of activation energy. Catalysts increase rate of reaction by providing an alternative pathway, which. Decreased activation energy means less energy required to start the reaction. This means that the reactant molecules have enough. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. as can. Explain Catalytic Action In Terms Of Activation Energy.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at a particular concentration, the rate. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. explain catalytic action in terms of activation energy. This does not. Explain Catalytic Action In Terms Of Activation Energy.
From www.cheric.org
Chemical Reaction (Reaction rate) Explain Catalytic Action In Terms Of Activation Energy When a catalyst is used at a particular concentration, the rate. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. a catalyst increases the rate of reaction by decreasing the activation energy. the activation energy of a reaction is 19.0 kj/mol. Catalysts increase rate of reaction by providing an. Explain Catalytic Action In Terms Of Activation Energy.
From www.numerade.com
Activation Energy and Catalysis The graph shows three reaction pathways Explain Catalytic Action In Terms Of Activation Energy This does not change the frequency of collisions. Decreased activation energy means less energy required to start the reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within. Explain Catalytic Action In Terms Of Activation Energy.
From exoddixpp.blob.core.windows.net
Define Catalyst Science Term at Michael Moorehead blog Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. This means that the reactant molecules have enough. explain catalytic action in terms of activation energy. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. This does not change the frequency of collisions. a substance that modifies the transition. Explain Catalytic Action In Terms Of Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Explain Catalytic Action In Terms Of Activation Energy explain catalytic action in terms of activation energy. a substance that modifies the transition state to lower the activation energy is termed a catalyst; a catalyst increases the rate of reaction by decreasing the activation energy. the activation energy of a reaction is 19.0 kj/mol. Catalysts increase rate of reaction by providing an alternative pathway, which.. Explain Catalytic Action In Terms Of Activation Energy.
From www.slideserve.com
PPT Energy and the Underlying Organization of Life PowerPoint Explain Catalytic Action In Terms Of Activation Energy a substance that modifies the transition state to lower the activation energy is termed a catalyst; the activation energy of a reaction is 19.0 kj/mol. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. Catalysts increase rate of reaction by providing an alternative pathway, which. the activation energy. Explain Catalytic Action In Terms Of Activation Energy.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Explain Catalytic Action In Terms Of Activation Energy as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. explain catalytic action in terms of activation energy. This means that the reactant molecules have enough. a catalyst increases the rate of reaction by decreasing the activation energy. the activation. Explain Catalytic Action In Terms Of Activation Energy.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. a catalyst increases the rate of reaction by decreasing the activation energy. explain catalytic action in terms of activation energy. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. When a catalyst is used at a particular concentration, the. Explain Catalytic Action In Terms Of Activation Energy.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. the activation energy is the minimum energy required for a reaction to occur. explain catalytic action in terms of activation energy. Decreased activation energy means less energy required to start the reaction. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants. Explain Catalytic Action In Terms Of Activation Energy.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Explain Catalytic Action In Terms Of Activation Energy This does not change the frequency of collisions. Catalysts increase rate of reaction by providing an alternative pathway, which. the activation energy of a reaction is 19.0 kj/mol. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. This means that the reactant molecules have enough. as can be seen. Explain Catalytic Action In Terms Of Activation Energy.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Explain Catalytic Action In Terms Of Activation Energy When a catalyst is used at a particular concentration, the rate. This means that the reactant molecules have enough. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. Decreased activation energy means less energy required to start the reaction. the activation energy of a reaction is 19.0 kj/mol. explain. Explain Catalytic Action In Terms Of Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Explain Catalytic Action In Terms Of Activation Energy This means that the reactant molecules have enough. Decreased activation energy means less energy required to start the reaction. the activation energy is the minimum energy required for a reaction to occur. the activation energy of a reaction is 19.0 kj/mol. Catalysts increase rate of reaction by providing an alternative pathway, which. activation energy is the minimum. Explain Catalytic Action In Terms Of Activation Energy.
From www.vrogue.co
The Catalyst Chemistry Resources For Teachers vrogue.co Explain Catalytic Action In Terms Of Activation Energy Catalysts increase rate of reaction by providing an alternative pathway, which. explain catalytic action in terms of activation energy. the activation energy of a reaction is 19.0 kj/mol. This does not change the frequency of collisions. This means that the reactant molecules have enough. a substance that modifies the transition state to lower the activation energy is. Explain Catalytic Action In Terms Of Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at a particular concentration, the rate. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. This means that the reactant molecules have enough. a catalyst increases. Explain Catalytic Action In Terms Of Activation Energy.
From schematiccntrylivinjhfxe.z21.web.core.windows.net
Energy Diagram Activation Energy Explain Catalytic Action In Terms Of Activation Energy When a catalyst is used at a particular concentration, the rate. This means that the reactant molecules have enough. Catalysts increase rate of reaction by providing an alternative pathway, which. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. This does not. Explain Catalytic Action In Terms Of Activation Energy.
From www.chim.lu
Activation energy and catalysis Explain Catalytic Action In Terms Of Activation Energy activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. a substance that modifies the transition state to lower the activation energy is termed a catalyst; Catalysts increase rate of reaction by providing an alternative pathway, which. the activation energy of a reaction is 19.0 kj/mol. This means that the. Explain Catalytic Action In Terms Of Activation Energy.
From www.sliderbase.com
Catalysis Presentation Chemistry Explain Catalytic Action In Terms Of Activation Energy a substance that modifies the transition state to lower the activation energy is termed a catalyst; the activation energy of a reaction is 19.0 kj/mol. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. a catalyst increases the rate. Explain Catalytic Action In Terms Of Activation Energy.
From saylordotorg.github.io
Catalysis Explain Catalytic Action In Terms Of Activation Energy This does not change the frequency of collisions. the activation energy is the minimum energy required for a reaction to occur. Decreased activation energy means less energy required to start the reaction. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. as can be seen from the arrhenius equation,. Explain Catalytic Action In Terms Of Activation Energy.
From slideplayer.com
For Foundation Chemistry Paper 2, you will need to revise ppt download Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. a catalyst increases the rate of reaction by decreasing the activation energy. Catalysts increase rate of reaction by providing an alternative pathway, which. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. as can be seen from the arrhenius. Explain Catalytic Action In Terms Of Activation Energy.
From studylib.net
Enzymes Lower Activation Energy Explain Catalytic Action In Terms Of Activation Energy as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. a catalyst increases the rate of reaction by decreasing the activation energy. Decreased activation energy means less energy required to start the reaction. the activation energy of a reaction is 19.0. Explain Catalytic Action In Terms Of Activation Energy.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Explain Catalytic Action In Terms Of Activation Energy a catalyst increases the rate of reaction by decreasing the activation energy. activation energy is the minimum energy needed for ‘successful collisions’ where atoms within the reactants rearrange to. This means that the reactant molecules have enough. Decreased activation energy means less energy required to start the reaction. When a catalyst is used at a particular concentration, the. Explain Catalytic Action In Terms Of Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Explain Catalytic Action In Terms Of Activation Energy This does not change the frequency of collisions. the activation energy is the minimum energy required for a reaction to occur. Decreased activation energy means less energy required to start the reaction. Catalysts increase rate of reaction by providing an alternative pathway, which. explain catalytic action in terms of activation energy. a catalyst increases the rate of. Explain Catalytic Action In Terms Of Activation Energy.
From online-learning-college.com
Catalysts What is a catalyst? How do they work? Explain Catalytic Action In Terms Of Activation Energy the activation energy of a reaction is 19.0 kj/mol. explain catalytic action in terms of activation energy. Decreased activation energy means less energy required to start the reaction. as can be seen from the arrhenius equation, the magnitude of the activation energy, \(e_a\), determines the value of the rate constant, \(k\), at a given. Catalysts increase rate. Explain Catalytic Action In Terms Of Activation Energy.